Abstract
Background
We fitted ordinal latent growth models to measures of drug availability to determine the trajectories of genetic and environmental effects over time.
Method
This report is based on data collected from 1789 adult males from the Mid Atlantic Twin Registry who participated in a structured telephone interview which included five retrospective assessments of drug availability between ages 8 and 25. Biometric univariate analysis revealed significant familial aggregation for all five measures of drug availability. In order to model latent growth, interval information (means and variance) was extracted from the ordinal data by simultaneously fitting a threshold invariant and multivariate Dual Change Score models. This permits the estimation of latent genetic and environmental trajectories over time.
Results
The relative proportions of genetic and environmental effects appear to be time dependent. There was a general increase in additive genetic and decrease in shared environmental effects over time. Stimulants were the only exception.
Conclusion
The upswing in genetic and specific environmental effects might be the result of acceleration in the expression of individual differences, and reductions in the total number of social constraints which may combine to make drugs easier to obtain.
Keywords: drug availability, gene, environment, twin, longitudinal, growth, ordinal
Introduction
“Drug availability is the most obvious environmental factor that influences addiction.” (Volkow and Li, 2005)
This study explores the genetic and environmental etiology of self-reported drug availability over time. The determinants of drug initiation are most likely complex and include heritable characteristics as well as environmental factors. Although a number of twin studies reveal that both genetic and environmental effects explain significant proportions of variance in the liability to initiate nicotine, alcohol, cannabis and other illicit substances (Heath and Martin, 1988; Prescott et al., 1994; Kendler et al., 1999; Kendler et al., 1999; Sullivan and Kendler, 1999; Rhee et al., 2003), they do not provide any information as to the quantitative trait loci (QTL) or putative environmental factors involved in initiation. In addition to searching for QTLs, the current goal should be to move beyond calculating heritabilities (basic genetic epidemiology paradigm), towards modeling developmental and environmental risk factors (advanced genetic epidemiology) contributing to drug initiation.
Two of the most obvious environmental risk factors for drug initiation include peer group deviancy and drug availability. Peer group deviancy (Dembo et al., 1979; Flay et al., 1994; Fergusson et al., 1995; Gaeta et al., 1998; Rose, 1998; Epstein et al., 1999; Hofler et al., 1999; Coffey et al., 2000; Sieving et al., 2000; Alexander et al., 2001; Prinstein et al., 2001; Griffin et al., 2002; von Sydow et al., 2002; Taylor et al., 2004; Slomkowski et al., 2005) and drug availability (Dembo et al., 1979; Hofler et al., 1999; Coffey et al., 2000; Alexander et al., 2001; Korf, 2002; Freisthler et al., 2005; Freisthler et al., 2005) are both significantly associated with licit and illicit drug initiation.
Despite inconsistent findings, biometrical genetic designs reveal that variation in self-report, parent and teacher rated measures of peer group deviancy is under both genetic and environmental influences (Iervolino et al., 2002; Walden et al., 2004; Cleveland et al., 2005; Saudino et al., 2005). Although not previously considered as heritable, it is nevertheless plausible that some variation in drug availability likewise has genetic and environmental components of variance.
Aim
The aim of this paper is therefore to explore, in a large population based sample of adult male twins, the relative contribution of genetic and environmental factors to individual differences in self-reported drug availability for alcohol, cigarettes, marijuana, cocaine and stimulants. To this end, we fit a biometric latent growth model to ordinal twin data, which permits us to examine the trajectories of genetic and environmental effects on drug availability over time.
Methods
Sample and assessment procedures
This report is based on data collected in the 3rd wave of interviews in a study of adult male twins from the Virginia Twin Registry. This registry is described in detail elsewhere (Prescott and Kendler, 1999; Kendler et al., 2003). Briefly, twins were eligible for participation in this study if one or both twins were successfully matched to birth records, were a member of a multiple birth with at least one male, were Caucasian, and were born between 1940 and 1974. Of 9,417 eligible individuals for the 1st wave (1993–1996), 6,814 (72.4%) completed the initial interviews. At least 1 year later, we contacted those who had completed the initial interview to schedule a 2nd interview wave. The 2nd interview (1994–1998) was completed for 5,629 (82.6%) of those who had completed the 1st interview. The 3rd interview wave was completed by 1796 male twins (84%) who completed the 2nd interview. This included 2 complete triplets which were excluded from the current analyses. Subjects were 24-62 years old (mean age = 40.3 years, SD = 9.0). Most subjects were interviewed by telephone. A small number were interviewed in person because of subject preference, residence in an institutional setting (usually jail), or not having telephone service.
After a full explanation of the research protocol, signed informed consent was obtained before all face-to-face interviews. Verbal assent was obtained before all telephone interviews. This project was approved by the Committee for the Conduct of Human Research at Virginia Commonwealth University. Interviewers had a Master’s degree in a mental health-related field or a Bachelor’s degree in this area plus two years of clinical experience. The two members of a twin pair were each interviewed by different interviewers who were blind to interview information about the co-twin.
During the 2nd interview an algorithm was developed to diagnose zygosity by genotyping 227 twin pairs with eight or more highly polymorphic DNA markers (Kendler et al., 2000). Using these pairs, we developed a Fisher discriminant function with PROC DISCRIM in SAS using height, weight, 6 standard zygosity questions, and the twins’ history of any blood tests. Using this discriminant function, we could confidently assign zygosity to the remainder of the sample (operationalized as an estimated probability of monozygosity of ≤10% or ≥90%) except for 97 pairs. Of these, we had usable DNA from both members of 65 pairs, from which we obtained zygosities. All available information on the remaining 32 cases, including photographs, was reviewed by 2 of us (K.S.K. and C.A.P.), who assigned final zygosities. Assignment of zygosity for twins without an interviewed cotwin was done using the discriminant function analysis.
This interview included retrospective assessments of drug availability for five categories of psychoactive substances: alcohol; cigarettes; cannabis; cocaine; and stimulants (speed, uppers). Subjects were asked to recollect drug availability at up to 4 age periods: 8 to 11, 12 to 14, 15 to 17, 18 to 21, and ages 21 to 25. Assessments of cigarette and alcohol availability were obtained only until 17 and 21 years respectively.
To increase the likely validity of this retrospective data, we used the life history calendar method (Furstenberg et al., 1987; Freedman et al., 1988; Kessler and Wethington, 1991). This method has demonstrated that although human memory is relatively poor at recall, it can be improved significantly when probed with careful questioning involving specific time periods and events. For each drug class, subjects were asked, “When you were [AGE] how easy would it have been to get [SUBSTANCE] if you wanted to use (it / them)?” Responses were recorded on a four point ordinal scale [“very difficult or don’t know”, “somewhat difficult”, “somewhat easy”, and “very easy”]. Our decision to combine “very difficult” and “don’t know” was based on the fact that during the pilot phase interviewers consistently noted that “don’t know” responses typically meant not knowing how to obtain a drug rather than not knowing about the drug or what it was. Moreover, true “don’t know” responses were very rare.
The current report is based on 1789 males with complete drug availability data. This included 749 complete (464 monozygotic and 285 dizygotic) and 291 incomplete (154 monozygotic and 137 dizygotic) twin pairs. Test-retest correlations (polychoric) for the drug availability measures are shown in Table 1. These are based on a sample of 141 subjects for whom the retest interval was 14-56 days (mean interval = 29 days). All correlations are adjusted for linear, quadratic and cubic effects of age at interview.
Statistical methods
Genetic analysis
Standard biometrical genetic model fitting methods were used (Neale and Cardon, 1992) whereby the total variance in each observed variable was decomposed into additive (A) genetic as well as shared (C) and unique (E) environmental variance. Since MZ cotwins are genetically identical, the additive genetic correlation is fixed to 1.0, whereas the DZ additive genetic twin correlation is 0.5 because they, on average, share only half their genes in common. An important assumption of this biometrical model is that shared environmental effects correlate to an equal extent in MZ and DZ twin pairs (Kendler et al., 1994). Non-shared environmental effects are by definition uncorrelated and also reflect measurement error including short-term fluctuations.
Latent Growth Modelling
We fitted latent growth models to estimate the trajectory of genetic and environmental effects that impact on variation in drug availability over time. In addition to estimating an initial starting value or intercept, this method examines the rate of change over time, or slope of the curve. Since first introduced by Vandenberg and Falkner (1965), latent growth approaches have since been developed to incorporate dynamic properties of the data through the use of latent difference scores (McArdle, 1986; McArdle et al., 2002; McArdle and Hamagami, 2003). This method is described in detail below.
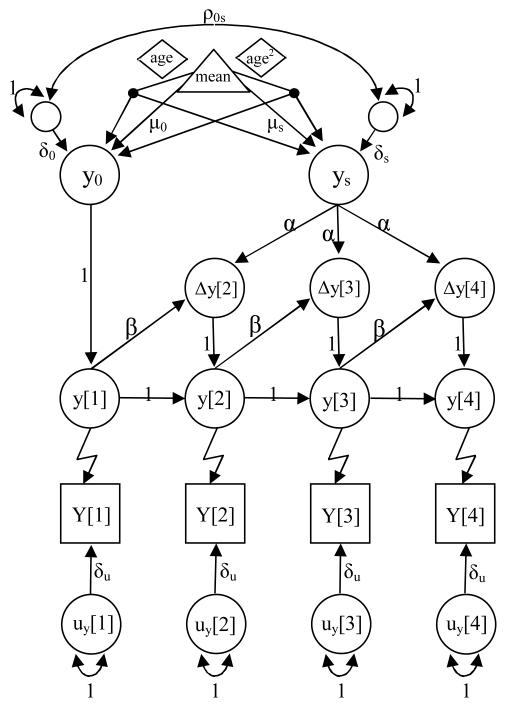
As shown Figure 1, let us assume that we have a series of observed scores (shown as squares) over four time points. Each observation has a corresponding latent, or unobserved true score (circles, y[1-4]) plus a unique error component (uy[1-4]). The initial level (y0) and the growth rate (ys) are also modeled as latent factors, along with their corresponding means (μ0, μs) and variances (δ0, δs), and latent difference scores (Δy[2-4]) are included. These parameters describe an individual’s trajectory as the summation of latent factor effects (y0, ys), i.e., accumulation of first differences among latent variables. An observed score at a particular time point [t] is therefore a linear combination of these components plus random error. This model is referred to as Dual Change Score (DCS) because there is a systematic change (α) and a systematic proportional change (β), both of which are assumed to be constant over time. In order to estimate the mean (μs) and variance (δs) of the latent slope score (ys), the systematic change (α) is fixed to 1. A purely systematic change model, where Δy= αysn is called a Constant Change Score (CCS) and is equivalent to the structured latent growth models first proposed by McArdle (1986). A purely systematic proportional change model, where Δy= βy[t-1]n, is called a Proportion Change Score (PCS) and is based on auto-regressive concepts developed in time series modeling (see Eaves et al., 1986). Because the CCS and PCS models are nested within the DCS, their goodness of fit can be judged using a chi-square statistic.
Figure 1.
Univariate Dual Change Score model.
Y0 = latent intercept, ys = rate of change, μ0, μs = latent intercept and slope means, δ0, δs = latent intercept and slope variances, ρ0s= correlation between intercept and slope, =Δy[2-4] = latent difference scores, uy[1-4] = random error, α = systematic change. Β = systematic proportional change. Also included are definition variables (diamonds) to adjust the mean (triangle) intercept and slope for the linear and quadratic effects of age at time of measurement. The sharp S-shaped single headed arrows represent the links between the observed ordinal measures (squares) and their corresponding underlying latent variables (circles).
To date, this DCS method has only been applied to continuous data. One approach to modeling ordinal data is to assume that there is an underlying continuous liability scale which is normally distributed in the population. The ordinal data then demarcate slices of this distribution separated by thresholds. Typically, the underlying distribution is assumed to have a mean of zero and variance of unity, and changes in the thresholds absorb any change in the underlying mean and variance. This approach prevents the estimation of parameters of models such as the DCS because the information about changes in mean and variance is lost. However, this problem can be overcome.
If we assume threshold invariance, whereby the ordinal data at different occasions of measurement are represented on a common metric, then it becomes possible to identify mean and variance changes. Mehta and colleagues (2004) have shown that by constraining the thresholds to be equal across time (i.e. threshold invariance), which effectively forces the time-standardized thresholds onto a common scale, the latent means and standard deviations can be freely estimated.
This common metric approach re-parameterizes the time-standardized thresholds in terms of thresholds on common or invariant scale, latent means on the newly defined common scale, and their standard deviations. The standard deviations are obtained by scaling the matrix of polychoric correlations among latent variables to a covariance matrix. A common metric can be obtained and estimated by fixing the first two thresholds (arbitrarily) to 0.0 and 1.0 respectively, at each time point. The third and any additional thresholds are estimated freely, but are also constrained to be equal over time. This forces the means and standard deviations to vary depending on changes in item endorsement. Note that it is possible to allow these free threshold parameters (which only exist when at least 4 ordinal categories are present in the data) to vary across time. Doing so provides a partial test of the assumption of measurement invariance.
In order to model latent growth, we fitted the DCS and nested CCS and PCS models to the ordinal data. In each case, the DCS provided a better fit to the data when compared to either of the nested models. In order to estimate the genetic and environmental trajectories, we then fitted a biometrical DCS model to twin data by the method of Maximum Likelihood (ML) using Mx (Neale, 1999) and decomposed the variance of the latent intercept and slope factors into genetic and environmental components.
Results
Item response frequencies for each drug class are reported in Table 2. Across all substances, drug availability increases i.e. it becomes reportedly easier to obtain with age.
Based on our model of threshold invariance, the means, standard deviations, common scale thresholds, and the model implied correlations for each drug class were estimated. These are shown in Table 3. The re-parameterization assumes that the thresholds on the new common scale can be anchored at the same place over time. This assumption was tested by comparing the unrestricted threshold model to the model of threshold invariance (for calculating degrees of freedom see Mehta et al., 2004), and was satisfied for cigarettes (Δ-2LL2df=3.21), marijuana (Δ-2LL4df=1.94), and stimulants (Δ-2LL1df=5.10), but not for alcohol (Δ-2LL3df=10.99) and cocaine (Δ-2LL4df=12.17). Yet despite the significant change in chi-square for alcohol, the model derived thresholds were very similar to the threshold estimates under the assumption of multivariate normality. Across all five substances, there was an overall increase in reported mean drug availability accompanied by a decline in the variance as drugs became reportedly easier to obtain.
Because we are analyzing non-independent twin data, we also tested a model which allowed for correlated residuals (one correlation for MZ and DZ twin pairs) which we then compared to a more parsimonious restricted model without correlated residuals. The restricted model provided a better fit for alcohol (Δ-2LL2df=0.22), cigarettes (Δ-2LL2df=2.58), stimulants (Δ-2LL2df=1.34), but not for marijuana (Δ-2LL2df=) and cocaine (Δ-2LL2df=7.94).
The decline in variance over time is represented graphically in the top portion of Figures 2 to 6 which show the un-standardized proportions of genetic and environmental variance over time. For all drug classes there is a sharp decline in the total variance between times 1 and 2. There is no notable increase in un-standardized variance except for alcohol availability between 18 and 21 years.
Figure 2.
Unstandardized and standardized proportions of variance in CIGARETTE availability. Variance components include latent genetic and environmental effects attributable to intercept and slope factors in he full biometrical DCS model.
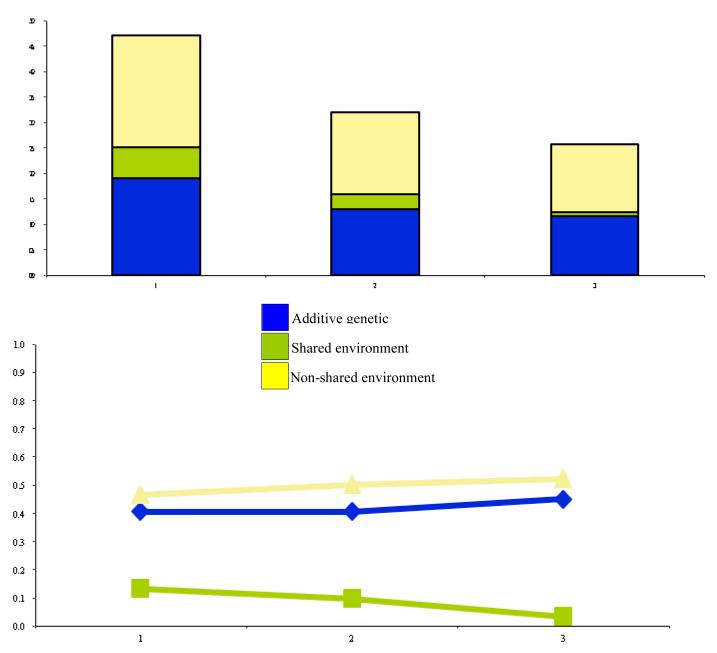
Figure 6.
Unstandardized and standardized proportions of variance in STIMULANT availability. Variance components include latent genetic and environmental effects attributable to intercept and slope factors in the full biometrical DCS model.
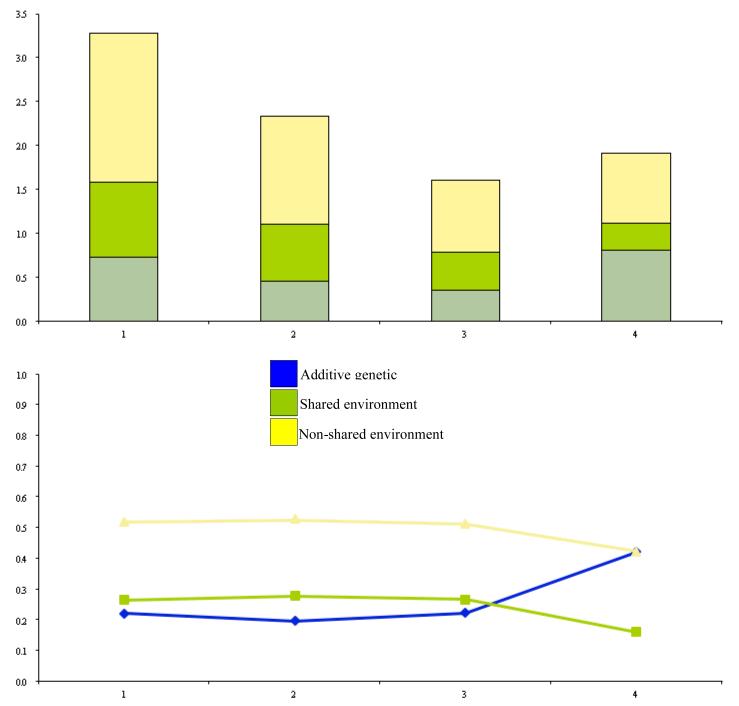
The standardized genetic and environmental trajectories are plotted in the lower halves of Figures 2 to 6. For all substances, non-shared environmental effects explained most of the standardized variance, and with the exception of stimulants, were longitudinally stable. Starting with cigarette availability, there was a very modest increase in additive genetic variance and decrease in share-environmental variance over time, while the non-shared environmental variance remained fairly stable. Across the five time points, the non-shared environment explained approximately 50% of the variance of alcohol availability. Although shared environmental effects initially explained more variance compared to genetic influences, at 18 years there was a cross-over and upswing in variance in the latter. A similar, but less marked trend was seen for marijuana. Shared environmental effects peaked between 12 and 17 years, but then began to decline slowly in favour of additive genetic variance. For cocaine, additive genetic effects accounted for very little variance between 8 and 17 years, after which time there begun a steady incline. Stimulants confounded all the previous trends. Additive genetic action accounted for more of the variance between 8 and 17 years, at which point there was a cross-over and upswing in the variance explained by shared environmental variance.
Discussion
For cigarette, alcohol, marijuana, and cocaine availability, a common feature for the genetic and environmental trajectories was the overall increase in additive genetic variance and decline in shared environmental variance over time. Non-shared environmental variance remained steady. Stimulant availability was the only drug class which did not follow this pattern. One possibility for this difference is that Ritalin is widely prescribed to school-aged children. Rather than being abused for its euphoric properties, it is possible that subjects abuse stimulants for the beneficial effects on academic performance. Therefore quite different risk factors affect its use than those that influence more recreational substances.
Geographical, social, and economic circumstances are all likely to influence accessibility to drugs. The shared environmental variance, particularly between 8 and 17 years, might be attributable to greater parental and social control. Examples of this would include within family effects such as parental styles and attitudes, schooling, church attendance and religious affiliation. The upswing and cross-over in additive genetic variance, most notably for alcohol between 18 and 21 years, coincides with acceleration in the expression of individual differences brought on by an increase in personal freedom and reduction in social constraints. An obvious example is the transition from home to college living which is accompanied by a dramatic increase in the likelihood of using marijuana and other forms of illicit substances (Gledhill-Hoyt et al., 2000).
Although the precise mechanisms for these shifts in variance components over time remain unknown, we can still speculate. For example, the observed trajectories may represent a shift from negative to positive genotype-environment correlation (CorGE). CorGE describe situations in which individual environments are unlikely to be entirely random but are partly caused by, or correlated with genotypes (Eaves et al., 1977; Neale and Cardon, 1992). These correlations arise because individuals either create (positive) or evoke (negative) environments which are a function of their genotypes (Eaves et al., 1977; Plomin et al., 1977; Scarr and McCartney, 1983).
Evocative or negative CorGE is related to Cattell’s (1963) principle of cultural coercion. The large shared environmental component may in part be a social response which coerces individuals who are predisposed to extreme behaviour, in either direction, towards the mean. It is conceivable that social coercion could limit awareness of drug availability in genetically susceptible individuals via drug interdiction, education, and threat of social sanction. However, it cannot explain how and why awareness of drug availability ought to increase among individuals at the extreme lower end of the distribution if a two-way movement towards the mean is assumed.
A more parsimonious explanation is that the observed shared environmental variance is exerted independent of genotypic variation, and is simply the result of cultural or social influences. These same shared environmental influences underpinning drug availability might also explain those found in drug initiation.
On the other hand, the increase in additive genetic variation, seen at older ages when teenagers are leaving home, could be caused by positive CorGE. This form of CorGE has been likened to a smorgasbord model which views culture as having a wide variety of environments from which individuals make selections based on their genetic preferences (Neale and Cardon, 1992). As an example, a teenager at high genetic risk, in an unconstrained environment, might be more likely to seek out, become knowledgeable of drug availability, and ultimately create or expose him/herself to drug environments. Under this scenario, the observed genetic variance in drug availability may explain some of the genetic variance in drug initiation. Genetic control of exposure to the environment, and hence knowledge of availability, may ultimately increase the odds of initiating.
Positive and negative CorGE increase and decrease genetic variance respectively. Unfortunately, we have no way of knowing, based on univariate analysis of data from twin pairs reared together, which genetic effects act directly on the phenotype and which result from the action of environmental variation caused initially by genetic differences (Neale and Cardon, 1992).
Another form of CorGE can arise if the environment in which individuals report drug availability is provided by their biological relatives. It has been shown that adolescents’ inattentive behaviour when combined with exposure to parental smoking, increase the probability of smoking by as much as 38% (Barman et al., 2004). It is therefore conceivable that a high genetic disposition to become aware of drug availability, and later initiate, could be correlated with permissive home environments, both of which can be provided by the parents.
In attempting to unravel the complex etiology of drug availability, one final consideration is the possibility of a genotype-environmental interaction (G×E) (Mather and Jinks, 1982) or genetic control of sensitivity to the environment. Unlike CorGE, which reflects a non random distribution of environments amongst different genotypes, G×E describes the ways in which genes affect environmental sensitivity, or conversely, environments affect gene expression (Neale and Cardon, 1992). A change in the environment, coupled with GxE effects, may account for the cross-over and upswing in genetic variance. A similar change has been observed for personality and intelligence in late teenage years (DeFries and Plomin, 1978; LaBuda et al., 1986; Eaves et al., 1989; Eaves et al., 1999; Gillespie et al., 2004).
Limitations
This paper integrates McArdle and Hamagami’s (2003) Dual Change Score with Mehta’s (2004) threshold invariance model for longitudinal data. This has enabled us to fit latent growth curves to longitudinal ordinal measures of drug availability. Our findings must be interpreted in the context of at least six potential limitations. First, our data were restricted to white males born in Virginia. Previous analyses using the same data (Kendler et al., 2000) suggest that this sample does not differ from the general population in rates of psychopathologic conditions, including illicit substance use and that it is likely to be broadly representative of US men. With regard to sex differences, although rates of cannabis and forms of substance abuse are lower in females, the genetic and environmental pathways to abuse and dependence across a range of illicit substances appear to be the same for men and women (Agrawal et al., 2005). Similar results may therefore be expected for females.
Second, the sample’s demography is more rural than urban. Despite this, previous analysis of the same sample has shown that adult prevalence rates of drug use are very similar to those found in national adult surveys (Kendler et al., 2002).
Third, our data were collected retrospectively. Twins were asked to recollect drug availability, and this introduces the potential for recall bias and telescoping effects (Pickles et al., 1994). Retest correlations were highest for substances more easily obtained and lower for the more illicit substances such as cocaine and stimulant. Correlations declined with greater intervening time intervals. Despite this limitation, the retest correlations suggest that our form of life history data collection, which was developed from the interface of cognitive & survey methodology, does in fact ascertain reliable data. All of our analyses were corrected for the linear and quadratic effects of age at interview which effectively removes most potential cohort effects.
Fourth, we are more likely to have assessed perceived versus actual drug availability. Whereas the former will always be more subjective, actual drug availability will be more sensitive to drug prices and the number of sellers within a particular area (Lettieri et al., 1980). Therefore, if the same items were administered to a population with decriminalized access to marijuana, the prediction is one of reduced variation in drug availability for MZ and DZ twin pairs alike, and hence a higher proportion of variance attributable to C. It remains an empirical question as to whether actual versus perceived drug availability is a better of predictor of subsequent initiation and use. One study has shown that perceived availability is a significant predictor for cannabis, heroin, alcohol and tobacco use but not for LSD or non-prescribed tranquillizers (Smart, 1977).
Fifth, our model fitting was not exhaustive. Alternate approaches may include modelling additional non-linear rate-of-change latent factors, or testing the fit of particular trajectories such as Gompertz, logistic and exponential curves (see Neale and McArdle, 2000). In addition, future bivariate designs should be used to determine how much of the shared environmental variance in drug availability overlaps with the shared environmental variance in drug initiation. Currently we have data on parental bonding, monitoring and attitudes, peer group deviancy, personality and household drug use from the same twins in the current study. These are likely to have strong etiological importance and possibly share covariance with drug availability, and might go some way to explain the large proportion of shared environmental effects in drug availability.
Finally, the finding of increased genetic and decreased shared environmental influences on drug availability may reflect changes in the cohabitation of twin pairs. It is possible that there is greater concordance for college choice, and more frequent sharing of accommodation by MZ twin pairs than by DZ twin pairs. Thus, it is possible that the equal environments assumption of twin studies is violated when twins reach their college years, but not violated when they are at home. Measurements of cohabitation history and geographical location of the twins would provide the opportunity to test this hypothesis.
Conclusion
Van Etten and Anthony (1999) have argued that understanding early stages of drug involvement is vital for epidemiological studies, because this in turn enables a better determination of whether variations in later substance use, abuse and dependence are related to differences in factors influencing initiation. As an example, the higher prevalence of drug use among males is more likely attributable to increased exposure and opportunities for use rather than to greater chance of progressing from initial opportunity to actual use (Van Etten et al., 1999). Improved epidemiology also facilitates evaluation of the efficacy of strategies and laws focussed on the prevention or delay of substance use, as well as policies aimed at the control of illicit substances.
The present paper has investigated, via latent growth curve modelling, the genetic and environmental trajectories of drug availability. We believe this is important in developing our understanding of drug initiation and escalation to use and abuse. A logical next step is to model, in a bivariate biometrical dual change system (see McArdle and Hamagami, 2003), the longitudinal relationship between drug availability and peer group deviancy. Such bivariate analysis will enable us to determine whether latent changes in peer group deviancy are causally related to latent changes in drug availability, or vice-versa, or whether common genetic and environmental factors better explain any covariation between deviancy and availability.
Figure 3.
Unstandardized and standardized proportions of variance in ALCOHOL availability. Variance components include latent genetic and environmental effects attributable to intercept and slope factors in the full biometrical DCS model.
Figure 4.
Unstandardized and standardized proportions of variance in MARIJUANA availability. Variance components include latent genetic and environmental effects attributable to intercept and slope factors in the full biometrical DCS model.
Figure 5.
Unstandardized and standardized proportions of variance in COCAINE availability. Variance components include latent genetic and environmental effects attributable to intercept and slope factors in the full biometrical DCS model.
Table 1.
Test-retest polychoric correlations drug availability measures based on 141 subjects.
| Cigarettes | 1. | 2. | 3. | 4. | 5. |
|---|---|---|---|---|---|
| 1. 8-11 | 0.78 | ||||
| 2. 12-14 | 0.71 | 0.78 | |||
| 3. 15-17 | 0.58 | 0.68 | 0.88 | ||
| Alcohol | |||||
| 1. 8-11 | 0.52 | ||||
| 2. 12-14 | 0.38 | 0.75 | |||
| 3. 15-17 | 0.26 | 0.52 | 0.65 | ||
| 4. 18-21 | 0.12 | −0.14 | 0.19 | 0.52 | |
| Marijuana | |||||
| 1. 8-11 | 0.40 | ||||
| 2. 12-14 | 0.52 | 0.78 | |||
| 3. 15-17 | 0.47 | 0.71 | 0.85 | ||
| 4. 18-21 | 0.16 | 0.49 | 0.73 | 0.75 | |
| 5. 22-25 | 0.13 | 0.20 | 0.42 | 0.54 | 0.62 |
| Cocaine | |||||
| 1. 8-11 | 0.32 | ||||
| 2. 12-14 | 0.30 | 0.41 | |||
| 3. 15-17 | 0.26 | 0.55 | 0.69 | ||
| 4. 18-21 | 0.28 | 0.49 | 0.65 | 0.75 | |
| 5. 22-25 | 0.10 | 0.20 | 0.50 | 0.67 | 0.79 |
| Stimulants | |||||
| 1. 8-11 | 0.36 | ||||
| 2. 12-14 | 0.49 | 0.45 | |||
| 3. 15-17 | 0.35 | 0.69 | 0.81 | ||
| 4. 18-21 | 0.08 | 0.38 | 0.71 | 0.73 | |
| 5. 22-25 | 0.28 | 0.16 | 0.65 | 0.66 | 0.71 |
Table 2.
Item response frequencies for each drug class by age.
| N | very difficult / don’t know |
somewhat difficult |
somewhat easy |
very easy | |
|---|---|---|---|---|---|
| Cigarettes | |||||
| 1. 8-11 | 1786 | 20.7 | 15.1 | 20.4 | 43.6 |
| 2. 12-14 | 1788 | 6.8 | 12.0 | 24.3 | 56.9 |
| 3. 15-17 | 1786 | 1.9 | 4.1 | 15.4 | 78.4 |
| Alcohol | |||||
| 1. 8-11 | 1787 | 40.3 | 21.6 | 17.2 | 20.8 |
| 2. 12-14 | 1788 | 20.8 | 22.8 | 27.1 | 29.2 |
| 3. 15-17 | 1788 | 5.9 | 11.1 | 30.9 | 52.0 |
| 4. 18-21 | 1788 | 0.7 | 1.7 | 8.6 | 88.4 |
| Marijuana | |||||
| 1. 8-11 | 1787 | 87.8 | 6.2 | 3.5 | 2.4 |
| 2. 12-14 | 1788 | 64.4 | 16.4 | 10.7 | 8.4 |
| 3. 15-17 | 1789 | 35.8 | 19.2 | 21.0 | 24.0 |
| 4. 18-21 | 1781 | 16.4 | 14.3 | 24.4 | 44.4 |
| 5. 22-25 | 1788 | 13.4 | 14.3 | 26.0 | 46.2 |
| Cocaine | |||||
| 1. 8-11 | 1787 | 96.3 | 2.4 | 0.7 | 0.4 |
| 2. 12-14 | 1787 | 87.8 | 8.6 | 1.8 | 1.7 |
| 3. 15-17 | 1788 | 68.2 | 17.7 | 7.7 | 6.3 |
| 4. 18-21 | 1780 | 45.4 | 20.7 | 16.9 | 16.4 |
| 5. 22-25 | 1789 | 38.6 | 22.5 | 18.0 | 20.8 |
| Stimulants | |||||
| 1. 8-11 | 1785 | 93.1 | 4.2 | 1.5 | 1.0 |
| 2. 12-14 | 1787 | 83.3 | 9.6 | 4.1 | 2.9 |
| 3. 15-17 | 1788 | 63.4 | 17.4 | 10.8 | 8.3 |
| 4. 18-21 | 1780 | 39.9 | 22.1 | 18.1 | 19.5 |
| 5. 22-25 | 1789 | 35.0 | 24.8 | 17.8 | 22.4 |
Table 3.
Estimates of thresholds on a common scale, means, standard deviations, and the model implied correlations.
| Cigarettes | |||||
|---|---|---|---|---|---|
| Threshold | Age 8-11 | Age 12-14 | Age 15-17 | ||
| 1 | 0.00 | 0.00 | 0.00 | ||
| 2 | 1.00 | 1.00 | 1.00 | ||
| 3 | 1.26 | 1.26 | 1.26 | ||
| M | 1.91 | 2.62 | 3.68 | ||
| SD | 2.33 | 1.81 | 1.77 | ||
| Age 8-11 | 1.00 | ||||
| Age 12-14 | 0.86 | 1.00 | |||
| Age 15-17 | 0.67 | 0.81 | 1.00 | ||
| Alcohol | |||||
| Threshold | Age 8-11 | Age 12-14 | Age 15-17 | Age 15-17 | |
| 1 | 0.00 | 0.00 | 0.00 | 0.00 | |
| 2 | 1.00 | 1.00 | 1.00 | 1.00 | |
| 3 | 1.18 | 1.18 | 1.18 | - | |
| M | 0.51 | 1.32 | 2.29 | 2.62 | |
| SD | 2.05 | 1.68 | 1.44 | 1.33 | |
| Age 8-11 | 1.00 | ||||
| Age 12-14 | 0.85 | 1.00 | |||
| Age 15-17 | 0.60 | 0.75 | 1.00 | ||
| Age 18-21 | 0.28 | 0.45 | 0.65 | 1.00 | |
| Marijuana | |||||
| Threshold | Age 8-11 | Age 12-14 | Age 15-17 | Age 15-17 | Age 18-21 |
| 1 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 2 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| 3 | 0.85 | 0.85 | 0.85 | 0.85 | 0.85 |
| M | 4.47 | 2.53 | 1.23 | 0.21 | 0.13 |
| SD | 2.25 | 1.82 | 1.76 | 1.69 | 1.56 |
| Age 8-11 | 1.00 | ||||
| Age 12-14 | 0.79 | 1.00 | |||
| Age 15-17 | 0.61 | 0.79 | 1.00 | ||
| Age 18-21 | 0.45 | 0.61 | 0.77 | 1.00 | |
| Age 22-25 | 0.39 | 0.46 | 0.61 | 0.78 | 1.00 |
| Cocaine | |||||
| Threshold | Age 8-11 | Age 12-14 | Age 15-17 | Age 15-17 | Age 18-21 |
| 1 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 2 | - | 1.00 | 1.00 | 1.00 | 1.00 |
| 3 | - | - | 0.91 | 0.91 | 0.91 |
| M | −3.94 | −1.80 | −0.87 | 0.18 | 0.49 |
| SD | 2.19 | 1.55 | 1.80 | 1.81 | 1.78 |
| Age 8-11 | 1.00 | ||||
| Age 12-14 | 0.81 | 1.00 | |||
| Age 15-17 | 0.64 | 0.80 | 1.00 | ||
| Age 18-21 | 0.46 | 0.63 | 0.80 | 1.00 | |
| Age 22-25 | 0.41 | 0.52 | 0.71 | 0.83 | 1.00 |
| Stimulants | |||||
| Threshold | Age 8-11 | Age 12-14 | Age 15-17 | Age 15-17 | Age 18-21 |
| 1 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 2 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| 3 | − | 0.89 | 0.89 | 0.89 | 0.89 |
| M | −3.26 | −1.94 | −0.65 | 0.42 | 0.61 |
| SD | 2.18 | 2.00 | 1.88 | 1.75 | 1.69 |
| Age 8-11 | 1.00 | ||||
| Age 12-14 | 0.81 | 1.00 | |||
| Age 15-17 | 0.66 | 0.84 | 1.00 | ||
| Age 18-21 | 0.51 | 0.65 | 0.79 | 1.00 | |
| Age 22-25 | 0.44 | 0.57 | 0.70 | 0.84 | 1.00 |
Acknowledgments
The project was supported by NIH grants DA-11287, MH/AA/DA-49492, MH-01458, and AA-00236, and NHMRC Sidney Sax Postdoctoral Fellowship. The authors thank Indrani Ray for database assistance. We thank Dr. Linda Corey for assistance with the ascertainment of twins from the Virginia Twin Registry, now part of the Mid-Atlantic Twin Registry (MATR), directed by Dr. Judy Silberg. The registry has received support from NIH, the Carman Trust, and the W.M. Keck, John Templeton, and Robert Wood Johnson Foundations.
References
- Agrawal A, Neale MC, Jacobson KC, Prescott CA, Kendler KS. Addictive Behaviors. 2005;30:1043–1048. doi: 10.1016/j.addbeh.2004.09.007. [DOI] [PubMed] [Google Scholar]
- Alexander C, Piazza M, Mekos D, Valente T. The Journal of Adolescent Health: Official Publication of the Society for Adolescent Medicine. 2001;29:22–30. doi: 10.1016/s1054-139x(01)00210-5. [DOI] [PubMed] [Google Scholar]
- Barman SK, Pulkkinen L, Kaprio J, Rose RJ. Addiction. 2004;99:1049–1061. doi: 10.1111/j.1360-0443.2004.00789.x. [DOI] [PubMed] [Google Scholar]
- Cattell RB. Journal of Educational Psychology. 1963;54:1–22. [Google Scholar]
- Cleveland HH, Wiebe RP, Rowe DC. The Journal of Genetic Psychology. 2005;166:153–169. [PubMed] [Google Scholar]
- Coffey C, Lynskey M, Wolfe R, Patton GC. Addiction. 2000;95:1679–1690. doi: 10.1046/j.1360-0443.2000.951116798.x. [DOI] [PubMed] [Google Scholar]
- DeFries JC, Plomin R. Annual Review of Psychology. 1978;29:473–515. doi: 10.1146/annurev.ps.29.020178.002353. [DOI] [PubMed] [Google Scholar]
- Dembo R, Farrow D, Schmeidler J, Burgos W. American Journal of Drug and Alcohol Abuse. 1979;6:313–336. doi: 10.3109/00952997909001721. [DOI] [PubMed] [Google Scholar]
- Eaves L, Eysenck HJ, Martin NG. Genes, Culture, and Personality: An Empirical Approach. Academic Press; London: 1989. [Google Scholar]
- Eaves L, Heath A, Martin N, Maes H, Neale M, Kendler K, Kirk K, Corey L. Twin Research. 1999;2:62–80. doi: 10.1375/136905299320565933. [DOI] [PubMed] [Google Scholar]
- Eaves L, Last KA, Martin NG, Jinks JL. British Journal of Mathematics and Statistical Psychology. 1977;30:1–42. [Google Scholar]
- Eaves LJ, Long J, Heath AC. Behavior Genetics. 1986;16:143–162. doi: 10.1007/BF01065484. [DOI] [PubMed] [Google Scholar]
- Eaves LJ, Martin NG, Eysenck SBG. British Journal of Mathematics and Statistical Psychology. 1977;30:185–197. [Google Scholar]
- Epstein JA, Botvin GJ, Diaz T. Archives of Pediatrics & Adolescent Medicine. 1999;153:1077–1084. doi: 10.1001/archpedi.153.10.1077. [DOI] [PubMed] [Google Scholar]
- Fergusson DM, Horwood LJ, Lynskey MT. Addiction. 1995;90:935–946. doi: 10.1046/j.1360-0443.1995.9079356.x. [DOI] [PubMed] [Google Scholar]
- Flay BR, Hu FB, Siddiqui O, Day LE, Hedeker D, Petraitis J, Richardson J, Sussman S. Journal of Health and Social Behavior. 1994;35:248–265. [PubMed] [Google Scholar]
- Freedman D, Thornton A, Camburn D, Alwin D, Young-demarco L. Sociological Methodology. 1988;18:37–68. [PubMed] [Google Scholar]
- Freisthler B, Gruenewald PJ, Johnson FW, Treno AJ, Lascala EA. Journal of Drug Education. 2005;35:15–27. doi: 10.2190/25QY-PBC3-B1EB-JB5Y. [DOI] [PubMed] [Google Scholar]
- Freisthler B, Needell B, Gruenewald PJ. Child Abuse and Neglect. 2005;29:1049–1060. doi: 10.1016/j.chiabu.2004.12.014. [DOI] [PubMed] [Google Scholar]
- Furstenberg FJ, Brooks-Gunn J, Morgan SP. Adolescent mothers in later life. Cambridge University Press; New York: 1987. [Google Scholar]
- Gaeta G, Del Castello E, Cuomo S, Effuso L, Pirera M, Boccalatte A. Cardiologia. 1998;43:417–426. [PubMed] [Google Scholar]
- Gillespie NA, Evans DM, Wright MJ, Martin NG. Twin Research. 2004;7:737–648. doi: 10.1375/1369052042663814. [DOI] [PubMed] [Google Scholar]
- Gledhill-Hoyt J, Lee H, Strote J, Wechsler H. Addiction. 2000;95:1655–1667. doi: 10.1046/j.1360-0443.2000.951116556.x. [DOI] [PubMed] [Google Scholar]
- Griffin KW, Botvin GJ, Scheier LM, Nichols TR. Substance Use & Misuse. 2002;37:225–238. doi: 10.1081/ja-120001979. [DOI] [PubMed] [Google Scholar]
- Heath AC, Martin NG. Alcoholism, Clinical and Experimental Research. 1988;12:735–741. doi: 10.1111/j.1530-0277.1988.tb01337.x. [DOI] [PubMed] [Google Scholar]
- Hofler M, Lieb R, Perkonigg A, Schuster P, Sonntag H, Wittchen HU. Addiction. 1999;94:1679–1694. doi: 10.1046/j.1360-0443.1999.941116796.x. [DOI] [PubMed] [Google Scholar]
- Iervolino AC, Pike A, Manke B, Reiss D, Hetherington EM, Plomin R. Child Development. 2002;73:162–174. doi: 10.1111/1467-8624.00398. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Jacobson KC, Prescott CA, Neale MC. American Journal of Psychiatry. 2003;160:687–495. doi: 10.1176/appi.ajp.160.4.687. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Karkowski, L M, Corey LA, Prescott CA, Neale MC. British Journal of Psychiatry. 1999;175:351–356. doi: 10.1192/bjp.175.4.351. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Karkowski LM, Neale MC, Prescott CA. Archives of General Psychiatry. 2000;57:261–269. doi: 10.1001/archpsyc.57.3.261. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Neale MC, Kessler RC, Heath AC, Eaves LJ. Psychological Medicine. 1994;24:579–590. doi: 10.1017/s0033291700027732. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Neale MC, Sullivan P, Corey LA, Gardner CO, Prescott CA. Psychological Medicine. 1999;29:299–308. doi: 10.1017/s0033291798008022. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Neale MC, Thornton LM, Aggen SH, Gilman SE, Kessler RC. Psychological Medicine. 2002;32:551–554. doi: 10.1017/s0033291701004950. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Wethington E. Psychological Medicine. 1991;21:723–738. doi: 10.1017/s0033291700022364. [DOI] [PubMed] [Google Scholar]
- Korf DJ. Addictive Behaviors. 2002;27:851–866. doi: 10.1016/s0306-4603(02)00291-5. [DOI] [PubMed] [Google Scholar]
- LaBuda MC, DeFries JC, Plomin R, Fulker DW. Child Development. 1986;57:1142–1150. doi: 10.1111/j.1467-8624.1986.tb00443.x. [DOI] [PubMed] [Google Scholar]
- Lettieri DJ, Mollie S, Pearson HW, editors. Theories on drug abuse: selected contemporary perspectives. U.S. Dept. of Health and Human Services, Public Health Service, Alcohol, Drug Abuse, and Mental Health Administration, National Institute on Drug Abuse, Division of Research; Washington, D.C.: 1980. [Google Scholar]
- Mather K, Jinks JL. Biometric Genetics: the study of continuous variation. 3rd ed. Chapman and Hall; London: 1982. [Google Scholar]
- McArdle JJ. Behavior Genetics. 1986;16:163–200. doi: 10.1007/BF01065485. [DOI] [PubMed] [Google Scholar]
- McArdle JJ, Ferrer-Caja E, Hamagami F, Woodcock RW. Developmental Psychology. 2002;38:115–142. [PubMed] [Google Scholar]
- McArdle JJ, Hamagami F. Behavior Genetics. 2003;33:137–159. doi: 10.1023/a:1022553901851. [DOI] [PubMed] [Google Scholar]
- Mehta PD, Neale MC, Flay BR. Psychological Methods. 2004;9:301–333. doi: 10.1037/1082-989X.9.3.301. [DOI] [PubMed] [Google Scholar]
- Neale MC. Mx: Statistical Modelling. 5ed. Department of Psychiatry; 1999. Box 126 MCV, Richmond, VA 23298. [Google Scholar]
- Neale MC, Cardon LR. Methodology for Genetic Studies of Twins and Families. Kluwer Academic Publishers: Dordrecht: 1992. [Google Scholar]
- Neale MC, McArdle JJ. Twin Research. 2000;3:165–177. doi: 10.1375/136905200320565454. [DOI] [PubMed] [Google Scholar]
- Pickles A, Neale M, Simonoff E, Rutter M, Hewitt J, Meyer J, Crouchley R, Silberg J, Eaves L. Behavior Genetics. 1994;24:457–468. doi: 10.1007/BF01076181. [DOI] [PubMed] [Google Scholar]
- Plomin R, DeFries JC, Loehlin JC. Psychological Bulletin. 1977;84:309–322. [PubMed] [Google Scholar]
- Prescott CA, Hewitt JK, Heath AC, Truett KR, Neale MC, Eaves LJ. J Stud Alcohol. 1994;55:18–33. doi: 10.15288/jsa.1994.55.18. [DOI] [PubMed] [Google Scholar]
- Prescott CA, Kendler KS. American Journal of Psychiatry. 1999;156:34–40. doi: 10.1176/ajp.156.1.34. [DOI] [PubMed] [Google Scholar]
- Prinstein MJ, Boergers J, Spirito A. Journal of Pediatric Psychology. 2001;26:287–298. doi: 10.1093/jpepsy/26.5.287. [DOI] [PubMed] [Google Scholar]
- Rhee SH, Hewitt JK, Young SE, Corley RP, Crowley TJ, Stallings MC. Archives of General Psychiatry. 2003;60:1256–1264. doi: 10.1001/archpsyc.60.12.1256. [DOI] [PubMed] [Google Scholar]
- Rose RJ. Alcohol Health and Research World. 1998;22:131–143. [PMC free article] [PubMed] [Google Scholar]
- Saudino KJ, Ronald A, Plomin R. Journal of Abnormal Child Psychology. 2005;33:113–130. doi: 10.1007/s10802-005-0939-7. [DOI] [PubMed] [Google Scholar]
- Scarr S, McCartney K. Child Development. 1983;54:424–435. doi: 10.1111/j.1467-8624.1983.tb03884.x. [DOI] [PubMed] [Google Scholar]
- Sieving RE, Perry CL, Williams CL. The Journal of Adolescent Health. 2000;26:27–35. doi: 10.1016/s1054-139x(99)00056-7. [DOI] [PubMed] [Google Scholar]
- Slomkowski C, Rende R, Novak S, Lloyd-Richardson E, Niaura R. Addiction. 2005;100:430–438. doi: 10.1111/j.1360-0443.2004.00965.x. [DOI] [PubMed] [Google Scholar]
- Smart RG. Bulletin on Narcotics. 1977;29:59–63. [PubMed] [Google Scholar]
- Sullivan PF, Kendler KS. Nicotine and Tobacco Research. 1999;1(Suppl 2):S51–57. doi: 10.1080/14622299050011811. discussion S69-70. [DOI] [PubMed] [Google Scholar]
- Taylor JE, Conard MW, Koetting O’Byrne K, Haddock CK, Poston WS. The Journal of Adolescent Health. 2004;35:190–196. doi: 10.1016/j.jadohealth.2004.01.008. [DOI] [PubMed] [Google Scholar]
- Van Etten ML, Anthony JC. Drug and Alcohol Dependence. 1999;54:117–125. doi: 10.1016/s0376-8716(98)00151-3. [DOI] [PubMed] [Google Scholar]
- Van Etten ML, Neumark YD, Anthony JC. Addiction. 1999;94:1413–1419. doi: 10.1046/j.1360-0443.1999.949141312.x. [DOI] [PubMed] [Google Scholar]
- Volkow N, Li TK. Nature Neuroscience. 2005;8:1429–1430. doi: 10.1038/nn1105-1429. [DOI] [PubMed] [Google Scholar]
- von Sydow K, Lieb R, Pfister H, Hofler M, Wittchen HU. Drug and Alcohol Dependence. 2002;68:49–64. doi: 10.1016/s0376-8716(02)00102-3. [DOI] [PubMed] [Google Scholar]
- Walden B, McGue M, Lacono WG, Burt SA, Elkins I. Journal of Abnormal Psychology. 2004;113:440–450. doi: 10.1037/0021-843X.113.3.440. [DOI] [PubMed] [Google Scholar]