Abstract
The postembryonic development of the gastrointestinal tract is subject to regulation by the colonizing microbiota. This maturation process requires the commensal bacteria to cross-talk with host cells by way of recognizing receptors and inducing signaling pathways to activate transcription factors such as the nuclear receptors. Here, we show that in colonic cell lines and in primary colonic cells, Enterococcus faecalis isolated from newborn babies possess the ability to regulate peroxisome proliferator-activated receptor-γ1 (PPARγ1) activity through phosphorylation. This results in elevated DNA binding and transcriptional activation of downstream target genes, including IL-10, a cytokine known to modulate innate immune function. Furthermore, phosphorylation appears tightly regulated as phospho-PPARγ1 becomes an immediate substrate for degradation possibly to curtail any extended transactivation. The involvement of PPARγ1 in a myriad of physiological processes further confirms that microflora-driven regulation might be important for a number of homeostatic strategies in the gut.
Keywords: microbe–host interaction, nuclear receptors, transcription
The peroxisome proliferator-activated receptor-γ (PPARγ) is a nuclear receptor involved in lipid metabolism, glucose homeostasis, inflammation, and regulation of cell specification (for a review, see ref. 1). There are two isoforms of PPARγ, γ1 and γ2, and each forms heterodimers with the retinoid X receptor (RXR), resulting in a complex that binds PPAR-responsive elements (PPREs) within target gene promoters. Ligands for PPARγ include naturally occurring fatty acids and their derivatives, along with the synthetic antidiabetic drugs thiazolidinediones (2).
PPARγ expression ranges from high levels in white and brown adipose tissue to moderate levels in macrophages, colon, kidney, and liver (3). Although PPARγ2 plays an important role in adipogenesis, PPARγ1 is the dominant form in virtually all other tissues. Interestingly, ligand activation of PPARγ1 alters cell morphology and the expression of genes consistent with a more differentiated phenotype in human colon cancer cells (4, 5).
The main function of the gastrointestinal tract is to supply the body with nutrients and energy to sustain satisfactory metabolism. The physiological mechanism is in part tuned by the interplay between the intestinal epithelium and resident gut microflora. Exposure to the microbiota immediately after birth is an event intimately coupled to dramatic changes in nutrient intake. Thus, the resident flora become an essential component of digestion necessary for the uptake of nutrients (reviewed in ref. 6). Moreover, colonization by the normal microbiota has been suggested to impose a tuning of the state of physiological inflammation to mount the necessary rapid response against incoming pathogens (7).
At least 27 nuclear receptors are expressed in the colon (8), of which PPARγ1 and liver receptor homolog (9) are strongly implicated in colon inflammation, whereas PPARγ and PPARδ may play opposing roles in colon cancer (reviewed in ref. 10). Given that the colon harbors ≈109 bacterial cells per gram (11), comprising 400 or so species, it is conceivable that our prokaryotic community has a direct impact on host protein function. This may be part of the basis for the establishment of gut homeostasis.
Our previous data indicating that PPARγ may participate directly in host–bacteria cross-talk, as well as the establishment of innate immune functions, made this nuclear receptor an ideal target for further mechanistic studies (12). A number of mechanisms by which PPARγ could be influenced by the resident flora could be envisaged. Second messengers in the form of bacterial components, or derivatives of such, could act as ligands for a number of different nuclear receptors. Activation of nuclear receptors through the induction of cell-surface receptors such as Toll-like receptors (TLRs) may be another mechanism.
Transcription factors are powerful regulators of gene function. Aberrant expression of prolonged activity results in drastic changes of cell fate that may result in perturbed gut homeostasis. Posttranslational modification is an important form of receptor modulation that impacts on function. One of these modifications, mostly studied in human PPARγ2, is the phosphorylation of a MAPK-dependent serine residue (Ser-114), which is reported to repress transcriptional activity of the receptor during adipocyte differentiation (13, 14). In stark contrast, the same MAPK-dependent phosphorylation also has been shown to activate PPARγ1-mediated transcription in other studies, implying a difference between PPAR isoforms or cell-type response to these variants (15, 16). Interestingly, the initiation of proteasomal degradation coupled to the phosphorylation of PPARγ adds to the complex picture reported for receptor regulation (17, 18).
Having established that commensal bacteria could influence PPARγ1 activity, we set out to assess whether receptor phosphorylation could be subject to bacterial regulation in colonic epithelium. In the present study, we have used a phospho-Ser-84/114-PPARγ-specific antibody to monitor changes in the phosphorylation status of the receptor. Our results show that a panel of Enterococci isolates from newborn babies, as well as specific ligands, induce phosphorylation of endogenous PPARγ1 and thus increase DNA binding and target gene expression, including IL-10. Furthermore, phosphorylated PPARγ1 rapidly becomes subject to protein degradation, a process that is restrained by proteasome inhibitors. Finally, in primary murine colonocytes, the presence of Enterococci appears to transiently retain the phosphorylated form of PPARγ1, akin to that seen when treated with proteasomal inhibitors. The importance of bacterial modulation of phospho-PPARγ1 offers a unique insight into one means of regulating colonic homeostasis during development.
Results
Synthetic Ligands Induce Phosphorylation of Endogenous PPARγ1 in HT-29.
To assess the impact of ligands on the induction of PPARγ phosphorylation, we monitored the protein phosphorylation status in the colonic adenocarcinoma cell line HT-29. Cells were stimulated with known PPARγ ligands (ciglitazone, pioglitazone, rosiglitazone, and troglitazone), along with the nonsynthetic ligand, 15-deoxy prostaglandin J2 (15d-PGJ2), all resulting in the phosphorylation of PPARγ1 (Fig. 1A). Pioglitazone was then used for both different durations and with varying concentrations to study the effect of PPARγ1 on phosphorylation. Concentrations as low as 50 nM yielded an altered phosphorylation pattern, and this phenomenon appeared to be PPARγ ligand-restricted because 100 μM PPARα-specific ligand WY-14643 did not have the same effect (Fig. 1B). Moreover, induced phosphorylation appeared within 30 min of stimulation, peaking at 1–2 h, without affecting the pool of total PPARγ (Fig. 1C). Notably, ligand-dependent phosphorylation was confirmed through the use of a ligand-binding inhibitor, GW9662, which efficiently prevented the phosphorylation of the receptor (Fig. 1D).
Fig. 1.
Ligands induce phosphorylation and promote binding of PPARγ1 in HT-29 cells. (A) Stimulation with the ligands pioglitazone (Pio), rosiglitazone (Rosi), troglitazone (Tro), ciglitazone (Ci), 15d-PGJ2, and WY-14643 (WY). (B) Western blot and quantifications of phospho-PPARγ relative to total PPARγ with different concentrations of pioglitazone, along with the PPARα-specific ligand WY-14643 (WY) at 2 h of stimulation. (C) Time course of phosphorylation caused by pioglitazone (Pio). (D) Stimulation with GW9662 (GW) and pioglitazone (Pio). All Western blots of nuclear extracts were sequentially probed with phospho-antibody (P-Pγ1) and total PPARγ antibody. All data are representative of at least five independent experiments.
Synthetic Ligands Mimic Bacterial Induction of Phosphorylation, Which Correlates to Increased DNA Binding of PPARγ1.
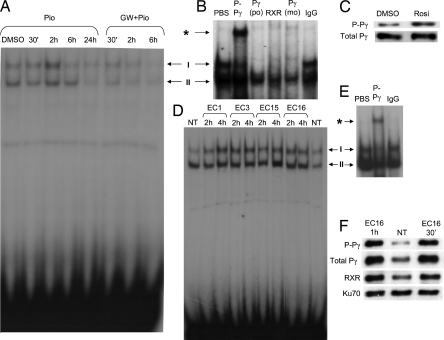
Having observed ligand-induced phosphorylation of PPARγ1, we next performed EMSAs by using a PPARγ-specific probe on nuclear extracts from ligand-treated HT-29 cells. Importantly, increasing the pool of phospho-PPARγ1 protein resulted in elevated DNA binding, which in turn was prevented by the PPARγ antagonist, GW9662 (GW+Pio; Fig. 2A). Furthermore, a monoclonal antibody recognizing Ser-84 phosphorylation of PPARγ1 supershifted the majority of complex I, whereas the total PPARγ antibodies depleted both DNA-binding complexes (Fig. 2B). Hence, changes seen in DNA binding would primarily represent changes due to phosphorylation of PPARγ1. Oligonucleotide pulldown using a biotinylated DR-1 element from the CYP450 gene was used to monitor the phospho-PPARγ levels bound to DNA. In Fig. 2C, the levels of phosphorylated PPARγ binding to DNA increased in extracts from cells cocultured with rosiglitazone. EMSA also was performed on nuclear extracts from HT-29s cocultured with different isolates of Enterococcus faecalis, which are early colonizers of the human gut. All four isolates (EC1, EC3, EC15, and EC16) seemed to affect DNA binding of PPARγ1 (Fig. 2D). We decided to focus on one of the isolates, EC16, and established its rapid but transient effect on receptor DNA binding (data not shown). We proceeded to show that phospho-PPARγ1 is a component of the altered DNA-binding complex in EC16-stimulated cells through supershift assay (Fig. 2E). The same increase as with ligand stimuli in DNA binding can be seen after EC16 stimulation (Fig. 2F). Here we also show the presence of the PPARγ-binding partner, RXR, confirming proper DNA complex formation, along with the nonspecific DNA-binding protein, Ku70, as a loading control.
Fig. 2.
Ligands and E. faecalis generate functional effects on DNA-binding PPARγ1 in HT-29 cells. (A) EMSA of pioglitazone (Pio) and GW9662 (GW) stimulation at different time points. Complexes I and II are as indicated. (B) Supershifts (*) by phospho-PPARγ (P-Pγ), total polyclonal PPAR-γ [Pγ (po)], and total monoclonal PPAR-γ [Pγ (mo)], together with control IgG antibodies. (C) Oligonucleotide pulldown of nuclear extracts from cells stimulated by rosiglitazone for 30 min. (D) EMSA with cocultured HT-29 nuclear extracts showing effects of four E. faecalis (all at 106) isolates (EC1, EC3, EC15, and EC16) on DNA-binding property of PPARγ1 at indicated time points. (E) Supershift by phospho-PPARγ (P-Pγ) and control IgG antibodies of nuclear extracts from HT-29 cocultured with EC16. (F) Phospho-PPARγ levels in oligonucleotide pulldown assay of nuclear extracts from HT-29s cocultured with EC16 and control (NT) for varying times as indicated. The PPARγ partner, RXR, is shown to establish the presence of a true receptor complex. The nonspecific DNA-binding protein, Ku70, was shown as a loading control. Data are representative of at least three independent experiments.
Increased Phospho-PPARγ1 Correlates to Increased Levels of Adipose Differentiation-Related Protein (ADRP), Fasting-Induced Adipose Factor (FIAF), and IL-10 Gene Expression.
Having demonstrated that resident flora from newborn children as well as synthetic ligands regulate the DNA binding of PPARγ1 in a phosphorylation-dependent manner, we next evaluated whether this phenomenon would correlate to the activation of downstream target genes by using SYBR Green-based real-time PCR. Time points of gene expression evaluation were set to 6 h to account for early transcriptional events. The effects on target gene expression were assessed, where the target gene, ADRP (or adipophilin), along with yet another PPARγ target gene, FIAF (or angiopoietin-like protein 4) (19, 20), were strongly induced by EC16 as well as a PPARγ-specific ligand, rosiglitazone (Fig. 3 A and B). In addition to ADRP and FIAF, we analyzed the effect of EC16 in IL-10, a cytokine essential for the maintenance of gut homeostasis. Here we show that IL-10 expression by HT-29 cells is increased when cocultured with both EC16 (Fig. 3C Left) and specific ligand (Fig. 3C Right). Furthermore, a decline in PPARγ protein levels, as achieved by specific siRNA knockdown experiments, indicated that EC16-induced FIAF expression is indeed dependent on PPARγ (Fig. 3D). The EC16-induced FIAF expression also was reviewed when testing the influence of the three different kinase inhibitors, PD98059, LY294002, and SB203580. This shows that the induction can be inhibited by LY294002 and partially by SB203580, whereas PD98059 seems unable to influence expression (Fig. 3E). HT-29s treated with EC16, together with either LY294002 or SBB203580, also show decreased levels of phospho-PPARγ1 (Fig. 3F), indicating that the PI3-kinase and, to some extent, the p38 MAPK pathways are instrumental in directing phosphorylation and, thus, activity.
Fig. 3.
PPARγ1 phosphorylation is coupled to increased transcription of target genes. (A) Real-time PCR experiment of ADRP under a 6-h influence of EC16 or rosiglitazone. (B) Real-time PCR of FIAF expression influenced by EC16 or rosiglitazone for 6 h. (C) Expressional changes of IL-10 in the presence of both rosiglitazone and EC16. (D) Inhibition by siRNA for PPARγ (SiPγ) on FIAF target gene expression compared with scrambled (Scr.) oligonucleotides. (E) Real-time PCR of FIAF in HT-29s treated with EC16 along with kinase inhibitors PD98059, LY294002, and SB203580 for 6 h. (F) Western blot of nuclear extracts of HT-29s stimulated by EC16 together with LY294002 or SB203580 for 30 min. Ku70 is shown as a loading control. Bars signify means with standard errors and are representative of at least three independent experiments.
Proteasomal Inhibition Sustains Phospho-PPARγ1 Levels and Prolongs DNA Binding of the Receptor Complex.
To establish whether the pool of phospho-PPARγ1 was influenced by degradation, HT-29 cells were subjected to the calpain inhibitor, ALLN, the 20S proteasome inhibitor, lactacystin, and a modifier of the proteasomal catalytic subunits, epoxomicin. All treatments led to the accumulation of the phosphorylated form of PPARγ1, differing only in the longevity of degradation inhibition (Fig. 4A). The amount of phosphorylated protein was elevated under the influence of MG-132 and even further so when costimulated with pioglitazone, which in turn could be inhibited by GW9662 (Fig. 4B). The disappearance of the phospho-PPARγ1-containing complex I in EMSAs at later time points (Fig. 2A) due to transient ligand effects can be impeded by MG-132 (Fig. 4C). PPARγ target gene transcription was subsequently assessed. Interestingly, the inhibition of the proteasome using MG-132 is enough to increase transcription; when costimulated with ligand, the response is synergistic. This synergism seen on ADRP expression as a result of MG-132 and ligand costimulation is effectively inhibited by GW9662, thus abrogating any ligand-specific effects (Fig. 4D). To further verify these findings, we performed similar stimulations on primary mouse colonocytes where EC16 and MG-132 both increase levels of phospho-PPARγ1 (Fig. 4E).
Fig. 4.
Proteasomal inhibition results in the retention of phospho-PPARγ1. (A) Western blot of treatment comparison among MG-132 (MG), ALLN, lactacystin (Lacta), and epoxomicin (Epox) on phospho- and total PPARγ1 after 6- and 24-h stimulation. (B) Western blot of nuclear extracts from HT-29 cells treated with MG-132 (MG), GW9662 (GW), and pioglitazone (Pio). (C) EMSA of stimulation with MG-132, GW9662, and pioglitazone. (D) Altered expression of ADRP after 6-h stimulation with combinations of GW9662, pioglitazone, and MG-132. Bars signify means with standard errors. (E) Whole-cell extracts of primary colonocytes from male NMRI mice were treated with EC16 or MG-132 for 1 h. Actin is shown as a loading control. Western blots were sequentially probed with phospho-PPARγ (P-Pγ1) and total PPARγ or Actin. Data are representative of at least three independent experiments.
Discussion
The colonization of the human gastrointestinal tract by microbes occurs almost immediately after birth. Here we demonstrate that a strain of E. faecalis (denoted as EC16), transferred from mother to child, can regulate and activate the transcription factor PPARγ1 in colonic cell lines as well as mouse primary colonic epithelial cells. This study reinforces the growing notion that microbiota contribute to mechanisms of homeostasis closely connected to postnatal endocrinological processes (reviewed in ref. 21).
The phosphorylation of PPARγ has been reported to exhibit both positive and negative outcomes on its transcriptional capacity (22). Our coculture experiments with a variety of isolates of E. faecalis from newborn children show that microbes can transiently affect the phosphorylation status of endogenous PPARγ long enough to trigger an activation of its downstream target genes. We find that the phospho-PPARγ–RXR complex may have an important role in the interplay between microflora and colonocytes. The phospho-PPARγ1 protein levels and the DNA binding correlate well with target gene expression levels in HT-29 cells, as demonstrated by real-time PCR. The importance of phosphorylation for the activation of transcription factors is widely acknowledged (23, 24). Recently, it was shown that the phosphorylation of PPARγ1 and PPARγ2 by cyclin-dependent kinase 9 increases their activity as part of adipocyte differentiation (25). Although a clear role for PPARγ in colonic epithelia has yet to emerge, our studies have attempted to place this receptor within the context of bacterial-induced gut homeostasis. Nevertheless, other receptors, including TLRs and NODs, are also vital to the maintenance of controlled inflammation and intestinal homeostasis (26).
PPARγ has been linked to a variety of physiological processes and different metabolic diseases (reviewed in ref. 27), and we and others have suggested a protective role of the receptor in the alimentary tract (28–30). Adachi et al. (29) convincingly showed that PPARγ expressed in epithelial cells is essential for protection against dextran sulfate sodium-induced colitis. We demonstrate that the PPARγ activation by EC16 and rosiglitazone can induce the expression of IL-10. Mice devoid of IL-10 spontaneously develop enterocolitis, which can be somewhat ameliorated through the use of rosiglitazone (31), suggesting a protective cross-talk between these two pathways. In our study, IL-10 mRNA induction by EC16 was evident already after 6 h, indicating this cytokine to be an important component in the postnatal regulation of inflammation. Selective species of E. faecalis have been shown to antagonize diarrhea induced by E. coli K88 through unknown mechanisms (32), but it is conceivable that not all strains of E. faecalis will have beneficial effects. Moreover, it was recently shown that the intestinal ecosystem is altered in cases of inflammatory reaction to stimuli in the host (33). Importantly, the composition of the microbiota is restored after dissipation of the inflammatory signal, a feature of a healthy homeostatic mechanism. Information as to how this reconstitution is organized may be gleaned from studies such as this one.
Nevertheless, detailed mechanisms of bacteria-driven PPARγ activation are still poorly understood. We have extended the observations for PPARγ by showing the effects of Ser-84 phosphorylation of endogenous PPARγ1 on transactivation and not only as a means for the ensuing degradation. Hence, increases in phosphorylation and subsequent degradation can both be regulated by bacterial presence. One possible set of mediators may be bacterial by-products. An earlier study provided evidence for the importance of PPARγ in directing the antiinflammatory activities of conjugated linoleic acid (CLA) (34). Because CLA is a microbial by-product, this finding was suggestive of a link between luminal bacteria and PPARγ function.
The input from gut flora is considerable given their role in influencing our postnatal development and quality of life. PPARγ has been shown to regulate metabolic target genes (35), including ADRP and FIAF, the levels of which increase in response to ligand and EC16. At present, we need to evaluate the components of this pathway, such as RXR, in mediating the response. Interestingly, both of these gene products modulate lipid accumulation in cells, a trait that has been linked to PPARγ-driven immunomodulation in dendritic cells (36). Moreover, IL-10 production in intestinal epithelial cells is partly dependent on the ligation of CD1d, a nonclassical MHC molecule that presents lipid antigens (37). PPARγ controls the expression of CD1d in dendritic cells (38), a function not yet determined in intestinal epithelial cells. These findings provide preliminary data linking lipid metabolism to the control of inflammation in epithelial cells. However, more experiments are required to demonstrate the molecular basis of this link.
The expression of these lipid metabolic factors also may be an important early postweaning mechanism to deal with a change in the nutritional milieu. It is therefore tempting to speculate that intestinal PPARγ1 may be one of several transcription factors controlling lipid metabolism, under the influence of microflora, recently reported in studies of obese patients (39). In an extended study, experiments in germ-free mouse models pointed toward a transmissible nature of obesity mediated by the microbiota (40). Hence, it is likely that the floral composition can alter metabolic potential through host-signaling pathways such as nuclear receptors.
Given that pathogenic bacteria have various means to subvert host cell responses (41, 42), it is conceivable that commensals and nonpathogenic microflora may possess mechanisms to regain homeostatic balance. It follows that if small changes of the functional status of microbiota could regulate potent factors such as nuclear receptors, one might envisage a targeted way of improving colonic ailments. Finally, the modulatory role of the gut microflora in both health and disease settings may open for new experimental designs to further our understanding of nuclear receptor activity in intestinal homeostasis.
Experimental Procedures
Cell Culture and Reagents.
The human colorectal adenocarcinoma cell line HT-29 (HTB-38; American Type Culture Collection) was grown and maintained according to the supplier's recommendations. Colons from male 10-week-old NMRI mice were scraped for epithelial cells and cultured in DMEM (Gibco).
All agonists and antagonists were dissolved in DMSO. Pioglitazone (5 μM), rosiglitazone (5 μM), troglitazone (5 μM), ciglitazone (5 μM), 15d-PGJ2 (10 μM), and GW-9662 (10 μM) were all acquired from Cayman Chemical. Cells were always pretreated with GW9662 for 1 h before further stimulation to avoid competition between compounds. The proteasome inhibitors MG-132 (10 μM), ALLN (25 μM), epoxomicin (100 nM), and lactacystin (5 μM) were all purchased from Calbiochem. The kinase inhibitors PD98059 (25 μM) and LY294002 (10 μM) were purchased from Calbiochem, while SB203580 (10 μM) came from Sigma–Aldrich. The PPARα ligand, WY-14643 (100 μM), was procured from Biomol. Antibodies for RXRα (sc-553), PPARγ (sc-7273 and sc-7196), and Actin (sc-1616) were obtained from Santa Cruz Biotechnology, and the anti-phospho-PPARγ-specific antibody, MAB-3632, was purchased from Chemicon.
The E. faecalis used here were isolates obtained from 1-month-old (EC1) and 3-day-old (EC3, EC15, and EC16) infants. Isolates were always added to cells at a concentration of 106.
EMSA.
DNA binding was assayed by using 5 μg of nuclear extracts in binding buffer consisting of 25 mM Hepes (pH 7.9), 70 mM KCl, 10% glycerol, 5 mM DTT, and 1 μg of polydIdC (Amersham) in the presence of 50,000 cpm of a radiolabeled oligonucleotide probe. The probe chosen was an element of high PPARγ-specificity and moderate affinity to ensure low involvement of additional PPARs (43). The element used was the ARE6 DR1 site of the mouse adipocyte lipid-binding protein, aP2, of the following sequence: 5′-TCTCTCTGGGTGAAATGTGC-3′, 5′-AGAGGCACATTTCACCCAGAGAGA-3′. Supershift assays were carried out by initial incubation of the extracts in binding buffer, together with 500 ng of the antibody of interest, as well as suitable controls, for 25 min at room temperature. Thereafter, polydIdC and probe were added to extracts and incubated for 30 min before gel electrophoresis.
Oligonucleotide Pulldown Assay.
To trap DNA-binding complexes for further investigation, we used an oligonucleotide pulldown assay. Custom biotinylated oligonucleotides of a high-affinity PPRE in the CYP4A6/P450 IV family [CYP4A6(Z)] were designed: 5′-Biotin-CTGAACTAGGGCAAAGTTCACAGT-3′, 5′-biotin-ACTGTGAACTTTGCCCTAGTTCAG-3′. These were coupled (1 μg per sample) to streptavidin-coated Sepharose beads (Amersham Biosciences) and used to pull down DNA-binding PPARγ-RXR heterodimers from 80 μg of nuclear extracts in a binding buffer consisting of 12% glycerol, 12 mM Hepes (pH 7.9), 4 mM Tris (pH 7.9), 150 mM KCl, 1 mM EDTA, 1 mM DTT, 10 μg poly (dIdC)/300 μg protein, 1.2 mM PMSF, and 1x Complete (Roche).
Real-Time PCR.
Total RNA was extracted by using an RNeasy mini-kit (Qiagen), and cDNA synthesis was performed by using SuperScript II (Invitrogen), both according to the manufacturer's instructions. We have designed and tested a selection of annotated and potential target genes for PPARγ by using SYBR Green (Applied Biosystems)-based semiquantitative real-time PCR. Primers were designed and tested according to Applied Biosystems recommendations: ADRP forward, 5′-CTGTTCACCTGATTGAATTTGC-3′, reverse, 5′-AGAGCTTATCCTGAGCATCCTG-3′; β-actin forward, 5′-CCTGGCACCCAGCACAAT-3′, reverse, 5′-gccgatccacacggagtact-3′; FIAF forward, 5′-AAAGAGGCTGCCCGAGAT-3′, reverse, 5′-TCTCCCCAACCTGGAACA-3′; IL-10 forward, 5′-TCCCTGTGAAAACAAGAGCA-3; reverse, 5-TGTCAAACTCACTCATGGCTTT-3′. The sample setups always included biological duplicates and experimental triplicates. Data are presented as fold change in the relative gene expression.
RNA Interference.
The siRNA for PPARγ and scrambled control was purchased from Dharmacon, along with the transfection reagent suitable for the HT-29 cell line (Dharmafect 4). Transfection efficiency was always confirmed through either Western blot or real-time PCR and was carried out at least 24 h before any further stimulation of cells.
Western Blot Analysis.
Cells treated according to figure specifications were harvested and lysed. Nuclear extracts were prepared according the nuclear extracts protocol of Schreiber (44), whereas whole-cell extracts were made with Schindler lysis buffer [50 mM Tris (pH 8), 0.1 mM EDTA, 0.5% Nonidet P-40, 10% glycerol, and 150 mM NaCl]. To prevent dephosphorylation in prepared extracts, 10 nM okadaic acid, 5 mM sodium fluoride, and 400 μM sodium vanadate were added, along with 1× Complete (Roche) and 1 mM PMSF. Immunodetection with an appropriate secondary peroxidase-conjugated antibody (DAKO) was followed by chemiluminescence (ECL; Amersham). Western blots were always sequentially probed with phospho-PPARγ, total PPARγ.
Quantification of protein bands was performed by using the Quantity One analysis software of the Discovery Series (Bio-Rad).
ACKNOWLEDGMENTS.
This work was supported by Cancerfonden, the Swedish Cancer Society; the Swedish Science Council; the Swedish Foundation for Strategic Research at the Strategic Research Center for Studies of Integrative Recognition in the Immune System, Karolinska Institute; and Vinnova, the Swedish Governmental Agency for Innovation Systems.
Footnotes
Conflict of interest statement: J.-A.G. is a shareholder, research grant receiver, and consultant of KaroBio AB.
References
- 1.Chinetti-Gbaguidi G, Fruchart JC, Staels B. Role of the PPAR family of nuclear receptors in the regulation of metabolic and cardiovascular homeostasis: New approaches to therapy. Curr Opin Pharmacol. 2005;5:177–183. doi: 10.1016/j.coph.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 2.Lehmann JM, et al. An antidiabetic thiazolidinedione is a high affinity ligand for peroxisome proliferator-activated receptor gamma (PPAR gamma). J Biol Chem. 1995;270:12953–12956. doi: 10.1074/jbc.270.22.12953. [DOI] [PubMed] [Google Scholar]
- 3.Fajas L, et al. The organization, promoter analysis, and expression of the human PPARgamma gene. J Biol Chem. 1997;272:18779–18789. doi: 10.1074/jbc.272.30.18779. [DOI] [PubMed] [Google Scholar]
- 4.Koeffler HP. Peroxisome proliferator-activated receptor gamma and cancers. Clin Cancer Res. 2003;9:1–9. [PubMed] [Google Scholar]
- 5.Sarraf P, et al. Differentiation and reversal of malignant changes in colon cancer through PPARgamma. Nat Med. 1998;4:1046–1052. doi: 10.1038/2030. [DOI] [PubMed] [Google Scholar]
- 6.Blaut M, Clavel T. Metabolic diversity of the intestinal microbiota: Implications for health and disease. J Nutr. 2007;137:751S–755S. doi: 10.1093/jn/137.3.751S. [DOI] [PubMed] [Google Scholar]
- 7.Sansonetti PJ. War and peace at mucosal surfaces. Nature Rev. 2004;4:953–964. doi: 10.1038/nri1499. [DOI] [PubMed] [Google Scholar]
- 8.Bookout AL, et al. Anatomical profiling of nuclear receptor expression reveals a hierarchical transcriptional network. Cell. 2006;126:789–799. doi: 10.1016/j.cell.2006.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coste A, et al. LRH-1-mediated glucocorticoid synthesis in enterocytes protects against inflammatory bowel disease. Proc Natl Acad Sci USA. 2007;104:13098–13103. doi: 10.1073/pnas.0702440104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arulampalam V, Greicius G, Pettersson S. The long and winding road to gut homeostasis. Curr Opin Gastroenterol. 2006;22:349–353. doi: 10.1097/01.mog.0000231806.65030.ed. [DOI] [PubMed] [Google Scholar]
- 11.Xu J, Gordon JI. Inaugural Article: Honor thy symbionts. Proc Natl Acad Sci USA. 2003;100:10452–10459. doi: 10.1073/pnas.1734063100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kelly D, et al. Commensal anaerobic gut bacteria attenuate inflammation by regulating nuclear-cytoplasmic shuttling of PPAR-gamma and RelA. Nature Immunol. 2004;5:104–112. doi: 10.1038/ni1018. [DOI] [PubMed] [Google Scholar]
- 13.Adams M, et al. Transcriptional activation by peroxisome proliferator-activated receptor gamma is inhibited by phosphorylation at a consensus mitogen-activated protein kinase site. J Biol Chem. 1997;272:5128–5132. doi: 10.1074/jbc.272.8.5128. [DOI] [PubMed] [Google Scholar]
- 14.Hu E, Kim JB, Sarraf P, Spiegelman BM. Inhibition of adipogenesis through MAP kinase-mediated phosphorylation of PPARgamma. Science. 1996;274:2100–2103. doi: 10.1126/science.274.5295.2100. [DOI] [PubMed] [Google Scholar]
- 15.Chana RS, Lewington AJ, Brunskill NJ. Differential effects of peroxisome proliferator activated receptor-gamma (PPAR gamma) ligands in proximal tubular cells: Thiazolidinediones are partial PPAR gamma agonists. Kidney Int. 2004;65:2081–2090. doi: 10.1111/j.1523-1755.2004.00624.x. [DOI] [PubMed] [Google Scholar]
- 16.Zhang B, et al. Insulin- and mitogen-activated protein kinase-mediated phosphorylation and activation of peroxisome proliferator-activated receptor gamma. J Biol Chem. 1996;271:31771–31774. doi: 10.1074/jbc.271.50.31771. [DOI] [PubMed] [Google Scholar]
- 17.Floyd ZE, Stephens JM. Interferon-gamma-mediated activation and ubiquitin-proteasome-dependent degradation of PPARgamma in adipocytes. J Biol Chem. 2002;277:4062–4068. doi: 10.1074/jbc.M108473200. [DOI] [PubMed] [Google Scholar]
- 18.Hauser S, et al. Degradation of the peroxisome proliferator-activated receptor gamma is linked to ligand-dependent activation. J Biol Chem. 2000;275:18527–18533. doi: 10.1074/jbc.M001297200. [DOI] [PubMed] [Google Scholar]
- 19.Ge H, et al. Differential regulation and properties of angiopoietin-like proteins 3 and 4. J Lipid Res. 2005;46:1484–1490. doi: 10.1194/jlr.M500005-JLR200. [DOI] [PubMed] [Google Scholar]
- 20.Mandard S, et al. The direct peroxisome proliferator-activated receptor target fasting-induced adipose factor (FIAF/PGAR/ANGPTL4) is present in blood plasma as a truncated protein that is increased by fenofibrate treatment. J Biol Chem. 2004;279:34411–34420. doi: 10.1074/jbc.M403058200. [DOI] [PubMed] [Google Scholar]
- 21.Hooper LV, Falk PG, Gordon JI. Analyzing the molecular foundations of commensalism in the mouse intestine. Curr Opin Microbiol. 2000;3:79–85. doi: 10.1016/s1369-5274(99)00055-7. [DOI] [PubMed] [Google Scholar]
- 22.Burns KA, Vanden Heuvel JP. Modulation of PPAR activity via phosphorylation. Biochimica et Biophysica Acta. 2007;1771:952–960. doi: 10.1016/j.bbalip.2007.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Decker T, Kovarik P. Serine phosphorylation of STATs. Oncogene. 2000;19:2628–2637. doi: 10.1038/sj.onc.1203481. [DOI] [PubMed] [Google Scholar]
- 24.Mayr B, Montminy M. Transcriptional regulation by the phosphorylation-dependent factor CREB. Nat Rev Mol Cell Biol. 2001;2:599–609. doi: 10.1038/35085068. [DOI] [PubMed] [Google Scholar]
- 25.Iankova I, et al. Peroxisome proliferator-activated receptor gamma recruits the positive transcription elongation factor b complex to activate transcription and promote adipogenesis. Mol Endocrinol. 2006;20:1494–1505. doi: 10.1210/me.2005-0222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abreu MT, Fukata M, Arditi M. TLR signaling in the gut in health and disease. J Immunol. 2005;174:4453–4460. doi: 10.4049/jimmunol.174.8.4453. [DOI] [PubMed] [Google Scholar]
- 27.Lehrke M, Lazar MA. The many faces of PPARgamma. Cell. 2005;123:993–999. doi: 10.1016/j.cell.2005.11.026. [DOI] [PubMed] [Google Scholar]
- 28.Dubuquoy L, et al. Impaired expression of peroxisome proliferator-activated receptor gamma in ulcerative colitis. Gastroenterology. 2003;124:1265–1276. doi: 10.1016/s0016-5085(03)00271-3. [DOI] [PubMed] [Google Scholar]
- 29.Adachi M, et al. Peroxisome proliferator activated receptor gamma in colonic epithelial cells protects against experimental inflammatory bowel disease. Gut. 2006;55:1104–1113. doi: 10.1136/gut.2005.081745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dubuquoy L, et al. PPARgamma as a new therapeutic target in inflammatory bowel diseases. Gut. 2006;55:1341–1349. doi: 10.1136/gut.2006.093484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lytle C, et al. The peroxisome proliferator-activated receptor gamma ligand rosiglitazone delays the onset of inflammatory bowel disease in mice with interleukin 10 deficiency. Inflamm Bowel Dis. 2005;11:231–243. doi: 10.1097/01.mib.0000160805.46235.eb. [DOI] [PubMed] [Google Scholar]
- 32.Garcia-Galaz A, Perez-Morales R, Diaz-Cinco M, Acedo-Felix E. Resistance of Enterococcus strains isolated from pigs to gastrointestinal tract and antagonistic effect against Escherichia coli K88. Rev Latinoam Microbiol. 2004;46:5–11. [PubMed] [Google Scholar]
- 33.Lupp C, et al. Host-mediated inflammation disrupts the intestinal microbiota and promotes the overgrowth of enterobacteriaceae. Cell Host Microbe. 2007;2:119–129. doi: 10.1016/j.chom.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 34.Bassaganya-Riera J, et al. Activation of PPAR gamma and delta by conjugated linoleic acid mediates protection from experimental inflammatory bowel disease. Gastroenterology. 2004;127:777–791. doi: 10.1053/j.gastro.2004.06.049. [DOI] [PubMed] [Google Scholar]
- 35.Berger JP, Akiyama TE, Meinke PT. PPARs: Therapeutic targets for metabolic disease. Trends Pharmacol Sci. 2005;26:244–251. doi: 10.1016/j.tips.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 36.Szatmari I, et al. PPARgamma regulates the function of human dendritic cells primarily by altering lipid metabolism. Blood. 2007;110:3271–3280. doi: 10.1182/blood-2007-06-096222. [DOI] [PubMed] [Google Scholar]
- 37.Colgan SP, Hershberg RM, Furuta GT, Blumberg RS. Ligation of intestinal epithelial CD1d induces bioactive IL-10: Critical role of the cytoplasmic tail in autocrine signaling. Proc Natl Acad Sci USA. 1999;96:13938–13943. doi: 10.1073/pnas.96.24.13938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Szatmari I, et al. PPARgamma controls CD1d expression by turning on retinoic acid synthesis in developing human dendritic cells. J Exp Med. 2006;203:2351–2362. doi: 10.1084/jem.20060141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: Human gut microbes associated with obesity. Nature. 2006;444:1022–1023. doi: 10.1038/4441022a. [DOI] [PubMed] [Google Scholar]
- 40.Turnbaugh PJ, et al. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027–1031. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- 41.Arbibe L, et al. An injected bacterial effector targets chromatin access for transcription factor NF-kappaB to alter transcription of host genes involved in immune responses. Nature Immunol. 2007;8:47–56. doi: 10.1038/ni1423. [DOI] [PubMed] [Google Scholar]
- 42.Finlay BB, McFadden G. Anti-immunology: Evasion of the host immune system by bacterial and viral pathogens. Cell. 2006;124:767–782. doi: 10.1016/j.cell.2006.01.034. [DOI] [PubMed] [Google Scholar]
- 43.Juge-Aubry C, et al. DNA binding properties of peroxisome proliferator-activated receptor subtypes on various natural peroxisome proliferator response elements. Importance of the 5′-flanking region. J Biol Chem. 1997;272:25252–25259. doi: 10.1074/jbc.272.40.25252. [DOI] [PubMed] [Google Scholar]
- 44.Schreiber E, Matthias P, Muller MM, Schaffner W. Rapid detection of octamer binding proteins with “mini-extracts,” prepared from a small number of cells. Nucleic Acids Res. 1989;17:6419. doi: 10.1093/nar/17.15.6419. [DOI] [PMC free article] [PubMed] [Google Scholar]