Abstract
Activation of cell division in the root apical meristem after germination is essential for postembryonic root development. Arabidopsis plants homozygous for a mutation in the ROOT MERISTEMLESS1 (RML1) gene are unable to establish an active postembryonic meristem in the root apex. This mutation abolishes cell division in the root but not in the shoot. We report the molecular cloning of the RML1 gene, which encodes the first enzyme of glutathione (GSH) biosynthesis, γ-glutamylcysteine synthetase, and which is allelic to CADMIUM SENSITIVE2. The phenotype of the rml1 mutant, which was also evident in the roots of wild-type Arabidopsis and tobacco treated with an inhibitor of GSH biosynthesis, could be relieved by applying GSH to rml1 seedlings. By using a synchronized tobacco cell suspension culture, we showed that the G1-to-S phase transition requires an adequate level of GSH. These observations suggest the existence of a GSH-dependent developmental pathway essential for initiation and maintenance of cell division during postembryonic root development.
INTRODUCTION
One of the most remarkable developmental features of higher plants is their capacity to generate new organs throughout their life cycle. Postembryonic development arises essentially from groups of highly organized, mitotically active cells called meristems. Meristems generate cells that will enter specific differentiation programs while simultaneously maintaining a population of proliferating, undifferentiated cells. A fundamental aspect of meristems is that they allow the supply of cells to be related to environmental constraints or demands, thereby permitting the fine-tuning of plant growth and development to the prevailing environmental conditions. The primary root and shoot meristems, which are located at the apex of the root and shoot, are established during embryogenesis (Laux and Jürgens, 1997), and they initiate postembryonic development after germination.
The Arabidopsis root presents a remarkably regular and simple radial structure in which single layers of defined cell number comprising the epidermis, cortex, endodermis, and pericycle surround the vascular tissues (Dolan et al., 1993). The cellular organization of the Arabidopsis root is first established during embryogenesis by specific divisions within the ground tissues (Scheres et al., 1994). After germination, the internal structure of the Arabidopsis root is maintained by nearly invariant patterns of cell division when grown under controlled conditions (Scheres et al., 1994). The simplicity of the Arabidopsis root and the amenability of this organism to genetic and molecular analysis have made it an excellent system in which to dissect the mechanisms controlling plant cell growth and differentiation.
Addressing the question of how cell division is controlled during developmental processes is fundamental to understanding plant development. Inherent in this question is the contribution of the cell cycle to plant development and morphogenesis. Indeed, direct insights into the role of the cell cycle in plant development have been obtained by modulating the expression of genes whose products are involved in the core cell cycle machinery. For example, roots of Arabidopsis overexpressing a mitotic cyclin showed an increase in cell number and a significant increase in root size, suggesting that cyclins could be a limiting factor during growth (Doerner et al., 1996). However, no alteration in the organization of the root was observed. A normally shaped root also was generated from a smaller than normal number of cells in tobacco plants expressing dominant-negative forms of the cell cycle–dependent Cdc2 kinase (Hemerly et al., 1995). Organ shape thus appears to be relatively insensitive to variations in cell number. Thus, cell division may contribute to growth in response to a higher level of regulation that controls meristem organization. Indeed, laser ablation experiments have demonstrated that positional signaling from surrounding cells is an essential aspect of cell identity and meristem organization in the Arabidopsis root (Van den Berg et al., 1995, 1997). However, the mechanisms controlling cell division during development remain largely unknown.
Genetic studies using Arabidopsis roots provide a powerful means to address the function of genes that regulate cell division during root growth and development, and numerous Arabidopsis mutants affected in root development are available. Some of these exhibit specific alterations in meristem organization and cell division patterns (reviewed in Scheres et al., 1996; Schiefelbein et al., 1997). A mutation in the ROOT MERISTEMLESS1 (RML1) gene, however, does not affect embryonic development but results instead in plants with an extremely short mature root composed of the same number of cells and cell files as the embryonic root (Cheng et al., 1995). The rml1 mutation does not affect axial and radial patterns of root cell organization, suggesting that the RML1 gene does not play an obvious role in cell fate specification. However, rml1 mutants fail to initiate cell division when germinated and, as a result, are unable to establish and maintain an active, undifferentiated meristematic zone in the root. Lateral and adventitious roots from callus of rml1 mutants have similar defects (Cheng et al., 1995). In contrast to the highly defective cell division phenotype of rml1 roots, cell division occurs in the apical shoot meristem, producing a small shoot with vegetative and sexual organs. Thus, the RML1 gene is required primarily for the regulation of cell division in root apical meristems (Cheng et al., 1995).
Here, we describe the cloning and sequence of the RML1 gene. Its coding sequence and functional analysis revealed that surprisingly, RML1 is allelic to CADMIUM SENSITIVE2 (CAD2) (Cobbett et al., 1998), encoding the previously described first enzyme of glutathione (GSH; γ-glutamylcysteinyl glycine) biosynthesis, γ-glutamylcysteine synthetase (γ-GCS; EC 6.3.2.2; May and Leaver, 1994); as a result all tissues of rml1 mutants are devoid of GSH. GSH is a ubiquitous tripeptide involved in cellular redox homeostasis that has been shown to be present in high concentrations in all plant tissues (May et al., 1998). We present evidence that GSH deficiency leads to a cell division block during the G1 phase. Furthermore, our results demonstrate that GSH is necessary for the initiation as well as maintenance of cell division. The role of GSH-dependent cell division in plant roots is discussed.
RESULTS
RML1 Is Allelic to CAD2, the Structural Gene for γ-GCS
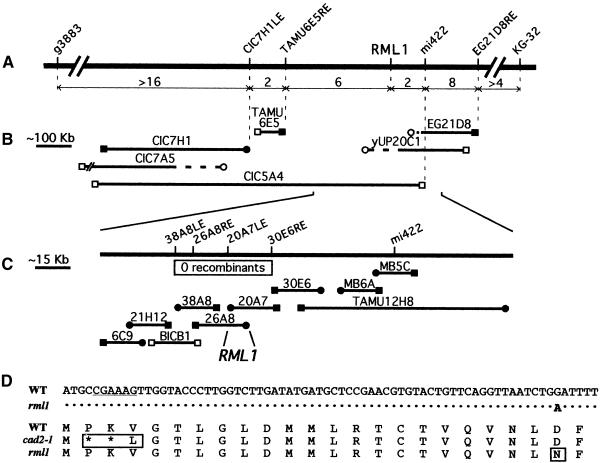
To study the molecular function of the RML1 protein, we devised a strategy to isolate the RML1 gene by using a restriction fragment length polymorphism (RFLP)–based chromosome walk technique, as shown in Figure 1. RML1 was mapped earlier to the middle of chromosome 4 between RFLP markers pCITd104 and AGAMOUS (AG) (Cheng et al., 1995). A yeast artificial chromosome (YAC) contig previously had been established between pCITd104 and AG on chromosome 4 (Schmidt et al., 1995), and RFLP probes derived from the ends of YAC clones in this region were used to analyze a segregating population of 605 plants. After having mapped RML1 between marker mi422 and the left end of marker CIC7H1 (CIC7H1LE), we deduced that the YAC clone CIC5A4 should span the gene (Figures 1A and 1B). Total DNA from the yeast strain harboring CIC5A4 was used to construct a cosmid library enriched for RML1 sequences in pCLD04541, a T-DNA–transferable vector that stably confers kanamycin resistance to transformed plants (Bent et al., 1994).
Figure 1.
Positional Cloning of the RML1 Gene.
(A) Genetic map of the RML1 region of chromosome 4 of Arabidopsis. The genetic map is represented by the open-ended solid line. The numbers over double-headed arrows indicate the distances between RFLP markers expressed as absolute numbers of recombinants in the F2 population. The regions between the left line break and marker g3883 and between the right line break and marker KG-32 are not drawn to scale.
(B) YAC (and bacterial artificial chromosome) contig. Clones anchored to the molecular markers are connected by dashed lines to the genetic map in (A). Solid circles denote right ends of clones; solid squares, left ends; and open squares, ends not aligned. YAC clones yUP20C1 and CIC7A5 are most likely chimeric clones (open circles connected to dashed lines). The region of CIC7A5 left of the line break is not drawn to scale.
(C) Cosmid walk from RFLP marker mi422 to RML1. All clones were constructed in the T-DNA–transformable cosmid vector pCLD04541 (Bent et al., 1994) from genomic DNA isolated from the yeast strain harboring YAC clone CIC5A4, except for the bacterial artificial chromosome clone TAMU12H8, which served to bridge the gap between clones MB6A and 30E6, and cosmid clone BICB1, isolated from an Arabidopsis genomic library kindly provided by K. Mayer (Institute of Plant Sciences, Swiss Federal Institute of Technology, Zurich, Switzerland). Symbols are the same as given in (B), and the box indicates the region for which no recombinants were detected using RFLP markers derived from the cosmid clones. The ∼10-kb overlapping sequence between complementing cosmids 20A7 and 26A8 was used to isolate cDNA clones encoding γ-GCS.
(D) Identification of the rml1 mutation. Sequence of the γ-GCS cDNA clone spanning nucleotides 820 to 890 and the encoded amino acid sequence spanning residues 236 to 259. The 6-bp deletion (824 to 829) mutation of the cad2-1 allele is underlined; this mutation yields a two–amino acid deletion (asterisks) and a one–amino acid substitution in the encoded protein (boxed). The single base pair (G to A) substitution mutation of rml1 is indicated by the letter A in boldface (dots indicate nucleotides identical to the wild-type [WT] sequence); this mutation gives rise to an aspartate to asparagine substitution in the encoded protein at residue 258 (boxed). The cysteine residue at position 251 is thought to be part of the active site of γ-GCS.
An overlapping cosmid contig was established by comparing patterns generated from restriction digestions and cross-hybridizations between cosmids and by segregation analyses (Figure 1C and data not shown). Six overlapping cosmids, of which four detected no recombinants in the segregating population, were mobilized into Agrobacterium for transformation of Arabidopsis plants that were heterozygous for the rml1-1 allele. Two cosmids, 20A7 and 26A8, which overlap by ∼10 kb, produced 86 transgenic T1 plants that were hemizygous for the T-DNA and heterozygous for the rml1 allele. These plants yielded only kanamycin-resistant T2 seedlings, which were all phenotypically wild type; thus, the 5- to 10-kb sequence shared between cosmids 20A7 and 26A8 was sufficient for complementation and therefore contained the RML1 gene.
A cDNA library was screened with the sequence shared between the complementing cosmids 20A7 and 26A8, and two cDNA clones were isolated. Both clones showed sequence identity with a previously published Arabidopsis sequence. The gene itself encodes the first enzyme of GSH biosynthesis, γ-GCS (May and Leaver, 1994). The cadmium-sensitive Arabidopsis mutant cad2-1 recently was also shown to be defective in the γ-GCS gene (Cobbett et al., 1998); however, cad2-1 cannot be morphologically distinguished from wild-type Arabidopsis. The roots of cad2-1 seedlings developed normally unless challenged by 0.5 to 1 μM Cd, in which case they turned brown (Howden et al., 1995). rml1 mutants died when germinated on medium containing 1 μM Cd, indicating an extreme sensitivity to Cd (data not shown).
To test for allelism, the homozygous cad2-1 mutant was crossed with rml1 heterozygotes. Given the normal root development in cad2-1, we expected that if it was allelic with rml1, the cad2-1 allele would complement the Rml1 stunted root, but not the Cd-sensitive phenotype, in cad2-1/rml1 plants. All plants in the F1 progeny grew normally, indicating that the Rml1 root phenotype was complemented. F1 plants were also tested for Cd sensitivity on medium containing 0.5 μM CdSO4 along with wild-type and cad2-1 controls. Approximately 50% of the progeny were Cd sensitive (rml1-1, 31 resistant to 39 sensitive,  , P > 0.5; rml1-2, 77 resistant to 77 sensitive,
, P > 0.5; rml1-2, 77 resistant to 77 sensitive,  , P > 0.9), indicating that the Cd-sensitive phenotype was not complemented. Collectively, these data indicate that cad2-1 and rml1 are allelic; thus, the rml1 plants are mutated in the γ-GCS gene.
, P > 0.9), indicating that the Cd-sensitive phenotype was not complemented. Collectively, these data indicate that cad2-1 and rml1 are allelic; thus, the rml1 plants are mutated in the γ-GCS gene.
The primers used to identify the cad2-1 mutation (Cobbett et al., 1998) were used to sequence the two rml1 alleles, rml1-1 and rml1-2 (Cheng et al., 1995). Surprisingly, both alleles harbored the same single G-to-A substitution mutation that encodes an asparagine residue at position 258 of the RML1 protein rather than an aspartate residue (Figure 1D), indicating that rml1-1 and rml1-2 are in fact the same allele. Therefore, throughout this article, we refer to rml1-1 and rml1-2 as the rml1 mutant and allele. The rml1 mutation is 60 bp from the cad2-1 mutation, which marks a 6-bp deletion resulting in the deletion of two amino acids at positions 237 and 238 and a valine-to-leucine substitution at position 239 in the encoded γ-GCS protein (Figure 1D; Cobbett et al., 1998).
The rml1 Mutant Is Deficient in γ-GCS Activity and as a Result Is Almost Devoid of GSH
To elucidate how the rml1 mutation affects protein function, we measured γ-GCS activity in rml1 and wild-type plants. As shown in Table 1, γ-GCS activity was undetectable in extracts of the rml1 mutant. Wild-type seedlings exhibited γ-GCS activity comparable with that observed previously (Cobbett et al., 1998). The activity of the second enzyme of GSH biosynthesis, GSH synthetase (GSHS), also was measured, and this activity essentially was not affected in rml1 mutants (Table 1). Quantification of the intracellular concentration of GSH was performed by using an HPLC assay (Cobbett et al., 1998). When compared with those of the wild type, extracts of rml1 mutants contained only 2.7% of the extractable GSH (Table 1). γ-Glutamylcysteine (γ-GC), the product of γ-GCS, was undetectable. In contrast, the amount of cysteine, one of the precursors of γ-GC, was approximately threefold higher than that in the extracts from wild-type plants. Importantly, confocal scanning laser microscopy analysis of whole rml1 seedlings labeled with the GSH-specific dye monochlorobimane (Sánchez-Fernández et al., 1997) revealed that the distribution of GSH in all organs was uniformally low when compared with the distribution seen in wild-type seedlings (data not shown). Thus, the rml1 mutation results in a reduction in the activity of γ-GCS in all cells to below the level of detection by using HPLC. The mutation leads to an extreme reduction in the intracellular pool of GSH and to a substantial increase in one of the precursors, cysteine.
Table 1.
Biochemical Characterization of Wild-Type and rml1 plants
| Lines | Cysteinea (nmol/g DW)b | γ-GCa (nmol/g DW) | GSHa (μmol/g DW) | γ-GCSa(nmol/min/mg Protein) | GSHSa(nmol/min/mg Protein) |
|---|---|---|---|---|---|
| Wild type | 109 ± 10 (100) | 60 ± 6 (100) | 3.02 ± 0.35 (100) | 0.80 ± 0.02 (100) | 0.98 ± 0.05 (100) |
| rml1 | 323 ± 19 (296) | NDc | 0.08 ± 0.01 (2.7) | ND | 0.88 ± 0.04 (90) |
The biochemical determination of thiols and of γ-GCS and GSHS activities was conducted with extract from 2-week-old liquid-grown plants subjected to HPLC as described in Methods. Numbers within parentheses indicate the percentage compared with the wild-type value.
DW, dry weight of tissue.
ND, undetectable.
Absence of Cell Division in the rml1 Postembryonic Root Results from the Depletion of Endogenous GSH
To investigate whether the root development phenotype of the rml1 mutant was directly linked to the impairment in GSH biosynthesis or to a pleiotropic effect of the mutation, we supplemented the growth medium with the product of γ-GCS activity, γ-GC, or its downstream anabolite GSH, to determine whether any of these substances could rescue root growth in the rml1 mutants. As described by Cheng et al. (1995), rml1 shoot tips can produce leaves, but cells in the root tip did not divide after germination, with the primary and secondary roots achieving a length of only ∼1 mm (Figure 2A). Importantly, rml1 could not be rescued when germinated on a medium containing glutamate and cysteine, the substrates of γ-GCS (data not shown). In contrast, when either 250 μM γ-GC or GSH was added to the growth medium before germination, the rml1 mutants developed roots up to 8 to 9 mm in length by 14 days after germination (Figures 2B and 2C). Because homozygous rml1 mutants exhibit reduced fertility, heterozygous parental strains segregating one-fourth rml1 plants were routinely used for these experiments.
Figure 2.
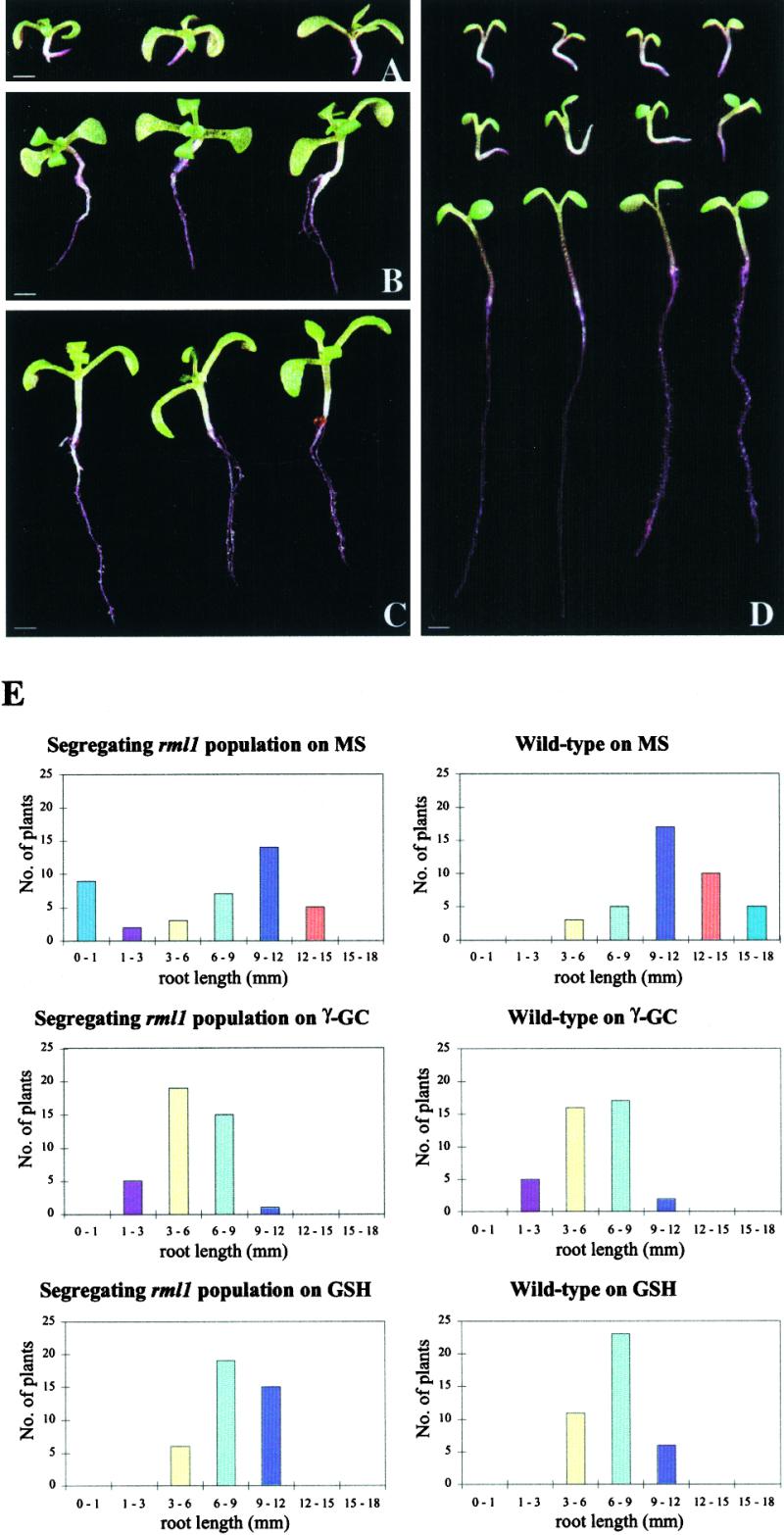
rml1 Rescue and Phenocopy.
(A) rml1 seedlings at 14 days after germination (DAG) grown on two-fifths MS medium. Note the short (∼1 mm) roots.
(B) rml1 seedlings at 14 DAG grown on two-fifths MS medium supplemented with 250 μM γ-GC.
(C) rml1 seedlings at 14 DAG grown on two-fifths MS medium supplemented with 250 μM GSH.
(D) Top, rml1 seedlings at 7 DAG grown on two-fifths MS medium; middle, wild-type seedlings at 7 DAG grown on two-fifths MS medium supplemented with 2.5 mM BSO, a specific inhibitor of γ-GCS; bottom, wild-type seedlings at 7 DAG grown on two-fifths MS medium. Note the similarity in root growth between the rml1 mutants and the wild-type plants grown on BSO. Wild-type plants develop relatively long roots at the same age when grown on BSO-free medium.
(E) Histograms show the results of a representative experiment in which wild-type and segregating rml1 populations (containing rml1/rml1:RML1/rml1:RML1/RML1 seeds in a 1:2:1 ratio) were germinated on MS medium or medium supplemented with 500 μM γ-GC or 750 μM GSH. The plants were grown vertically for 6 days before the root lengths were measured.
 .
.
At concentrations of 250 μM γ-GC or GSH, rml1 mutants in the segregating population could still be distinguished from wild-type and heterozygous plants. At higher γ-GC or GSH concentrations, the distribution of root lengths of segregating rml1 populations was indistinguishable from that of the wild type, whereas on Murashige-Skoog (MS) medium (Murashige and Skoog, 1962), the root lengths of the rml1 segregating population showed the expected 3:1 wild type plus heterozygote to rml1 mutant ratio (Figure 2E). In contrast, we could not rescue rml1 mutants with the oxidized form of GSH, GSSG, with ascorbate, another important antioxidant, or with DTT, a synthetic thiol (data not shown). Thus, exogenous GSH (and more precisely its reduced form) alleviated the block imposed on cell division by depleting the cellular GSH pool. Although growth is determined both by cell division and elongation, we do not know the exact role of GSH in processes regulating the elongation of root cells.
To determine whether GSH is required continuously during root growth, we grew rml1 mutants for 3 days on GSH-containing media and then transferred them to media lacking GSH. As shown in Table 2, we found that in the absence of exogenous GSH, root growth decreased rapidly and stopped totally after 2 days, whereas roots remaining on GSH-containing media grew 10 to 11 mm each day. Therefore, exogenous GSH was necessary not only for initiating but also for maintaining rml1 root growth.
Table 2.
Daily Root Growth of rml1 Plants
| Treatmenta | First Day (mm)b | Second Day (mm) | Third Day (mm) |
|---|---|---|---|
| + GSH | 11.3 ± 1.7 | 10.8 ± 1.7 | 11 ± 1.4 |
| − GSH | 3.5 ± 0.6 | 1.1 ± 0.2 | 0 |
Two-day-old rml1 seedlings were grown for 4 days on a medium supplemented with 250 μM GSH before being transferred onto a medium supplemented with 250 μM GSH (+ GSH) or lacking GSH (− GSH).
Numbers represent the mean of 12 roots ±sd.
To confirm further that GSH depletion was directly responsible for the absence of postembryonic growth in the rml1 root, we germinated wild-type Arabidopsis on media containing 2.5 mM l-buthionine-(S,R)-sulfoximine (BSO). BSO is a nontoxic and highly specific inhibitor of the first enzyme of GSH biosynthesis, γ-GCS, and its application results in the depletion of cellular GSH (Griffith and Meister, 1979; May and Leaver, 1993). As shown in the middle row of Figure 2D, wild-type seedlings exhibited growth similar to rml1 mutants (top row) by 7 days after germination on medium containing BSO. By comparison, wild-type seedlings grown in the absence of BSO developed long roots (Figure 2D, bottom row), confirming the direct link between the phenotype of the rml1 mutant and impairment in GSH biosynthesis. Thus, the depletion of intracellular GSH, either physiologically or genetically, inhibits postembryonic root growth in Arabidopsis. The addition of BSO to the growth medium of Arabidopsis seedlings inhibited root apical meristematic activity but allowed shoot meristematic activity to occur after germination. Like the control plants, 3-week-old rml1 mutants (Cheng et al., 1995) and BSO-treated wild-type plants produced six or seven leaves, although the leaves were somewhat shorter than those of plants grown in the absence of BSO (S. Muroy and Z.R. Sung, unpublished data).
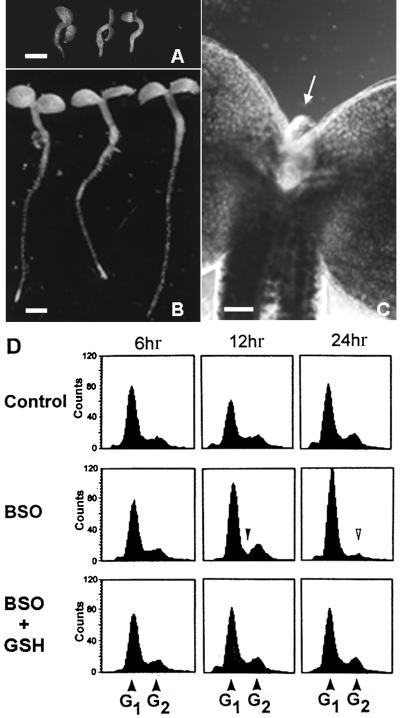
DNA Content of Cells in the Root Tip of rml1 Arabidopsis Mutants
The cloning and functional analyses of the rml1 mutant suggested a specific role for the antioxidant GSH in the control of root growth in the postembryonic root of Arabidopsis. Because root growth results from increases in cell number and cell length, and cell division arrest is the most dramatic phenotype in rml1 roots (Cheng et al., 1995), we investigated the possibility that GSH regulates the cell division cycle in the root apex. To elucidate the effect on cell division of the depletion of cellular GSH that occurs as a result of the rml1 mutation, nuclear DNA content of the tissue of rml1 mutants was analyzed by using flow cytometry, as shown in Figure 3. The ploidy distributions for nuclei isolated from the shoot tip were identical in 3-day-old rml1 and wild-type Arabidopsis plants (Figures 3A and 3B) and similar to that previously observed (Galbraith et al., 1991), confirming that cell division is not affected in rml1 shoots (Cheng et al., 1995). In the root tip of both rml1 and wild-type 3-day-old seedlings, different extents of polyploidy were observed (Figures 3C and 3D). The presence of polyploid cells in Arabidopsis roots did not allow us to identify a specific defect in the cell cycle arising from the rml1 mutation. However, we observed significant differences in the distribution of the nuclei in the root. For example, the 8C peak was predominant in rml1 plants, whereas the 4C peak was predominant in wild-type plants (Figures 3C and D). Moreover, the 32C population was undetectable in rml1 root tips (Figure 3C).
Figure 3.
Cellular DNA Content in rml1 and Wild-Type Plants.
DNA contents were analyzed by using flow cytometric analysis. The different ploidy levels are indicated.
(A) and (C) Extracts from rml1 shoots and root tips, respectively.
(B) and (D) Extracts from wild-type shoots and root tips, respectively.
Using a Tobacco Cell Suspension to Understand the Phenotype of the rml1 Mutant
To study specifically the effect of a GSH depletion on cell cycle progression, we sought to exploit a cell suspension system that could be synchronized. Because an Arabidopsis cell suspension that can be synchronized is currently unavailable, we used a highly specialized tobacco BY-2 cell suspension that has been widely adopted for cell cycle studies in plants (Nagata et al., 1992).
We first germinated tobacco plants on a medium containing 2.5 mM BSO. As observed with Arabidopsis plants, depletion of intracellular GSH in tobacco seedlings completely abolished root growth (Figures 4A and 4B), whereas shoot meristematic activity was essentially unaffected (Figure 4C). Therefore, depletion of intracellular GSH has similar effects on tobacco postembryonic root development, as seen with Arabidopsis plants, suggesting a general mechanism in plants.
Figure 4.
Using a Tobacco Cell Suspension to Determine the Phenotype of the rml1 Mutants.
(A) SR1 tobacco seedlings at 2 days after germination (DAG) grown on medium supplemented with 2.5 mM BSO.
(B) SR1 tobacco seedlings at 2 DAG grown on medium lacking BSO.
(C) Detail of the shoot apex of a 2-DAG SR1 tobacco seedling germinated and grown in the presence of 2.5 mM BSO and visualized on a Reichert Polyvar microscope (Leica, Heerbrugg, Switzerland) using Nomarski differential interference contrast optics. Note the developing leaf primordia (arrow).
(D) Tobacco BY-2 cell suspensions were treated with water (Control), 1 mM BSO, or as a further control to test the reversibility of the effects of BSO, 1 mM BSO and 100 μM GSH. The effects of depletion of intracellular GSH on cell cycle progression were followed by using flow cytometry. Depletion of S-phase cells is evident at 12 hr (solid arrowhead) and of G2-phase cells at 24 hr (open arrowhead). Similar results were obtained in three independent experiments.
 ;
;  .
.
We then checked whether GSH depletion could affect cell division in a BY-2 cell suspension culture. Exponentially growing BY-2 cells were treated with 1 mM BSO. From an initial value of 4.71 ± 0.09 μg (g dry weight)−1 of tissue, the cellular concentration of GSH progressively decreased to 0.21 ± 0.02 μg (g dry weight)−1 after 12 hr and to 0.11 ± 0.05 μg (g dry weight)−1 at 24 hr, while remaining stable in an untreated culture (data not shown). The effect of this treatment on cell cycle progression was studied by using flow cytometric analysis (Figure 4D). After 12 hr of BSO treatment, a pronounced depletion of the nuclei population during S phase was observed; after 24 hr, >95% of the nuclei from BSO-treated cells were in G1 phase compared with 60% in the control (Figure 4D). The almost total depletion of the G2 population after 24 hr of BSO treatment indicates that the cells remaining in G2 at 12 hr most probably represent cells that have not completed their cycle rather than cells arrested in G2 phase as a result of depletion of intracellular GSH. The cellular concentration of GSH was essentially the same at 12 and 24 hr. The effects of supplementing BSO with GSH yielded the same results when compared with the control plants (data not shown), demonstrating that the effects of BSO on cell cycle progression are directly linked to depletion of endogenous GSH. These data suggest that the mechanisms acting to block the cell cycle in roots when GSH is depleted also exist in tobacco BY-2 cells. Moreover, the G1-to-S transition appears to be a target for GSH-dependent control of progression through the cell cycle.
G1-to-S Phase Transition in Tobacco Cell Suspension Requires an Adequate Concentration of Intracellular GSH
Two possibilities might explain the G1 block induced by depletion of the intracellular GSH pool: either de novo synthesis of GSH is required at the G1-to-S transition, or more simply, there is a specific requirement for an adequate cellular concentration of GSH. To distinguish formally between these two possibilities, we analyzed the effects of an increase or a depletion of intracellular GSH on entry into S phase in highly synchronized BY-2 cells. The cells were treated either with exogenous GSH during the G1 phase or with different concentrations of BSO before entry into G1 to ensure a sufficient depletion of cellular GSH (see Methods). The effect of these treatments on cell cycle progression was followed by flow cytometric analysis, measurement of DNA synthesis, and the determination of the cellular concentration of GSH, as shown in Figure 5. Synchronized cell suspensions that had not been exposed to these treatments were used to establish the timing of cell cycle events. At time 0, all of the cells were in the beginning of G1 phase (data not shown). Three hours later, cells were at the G1-to-S transition; at 6 hr, cells were in mid-S phase; at 8 hr, they were in late S phase; and at 12 hr, they were in G2-to-M (Figure 5A). The cellular concentration of GSH increased slightly from 0 to 1 hr, remained stable until 6 hr, and began to increase only after 8 hr (Figure 5C).
Figure 5.
Entry into S Phase Is Regulated by the Cellular Concentration of GSH in Synchronized Tobacco BY-2 Cell Suspensions.
Synchronized tobacco BY-2 cells were treated with exogenous GSH (0.1 mM) at the beginning of G1, defined as time 0 of the experiment, or with BSO (0.1 or 1 mM) 2 hr before entry into G1 phase. Control cells were treated with water at time 0.
(A) Flow cytometric analysis of the cell cycle. Similar results were obtained in three independent experiments.
(B) DNA synthesis (3H-thymidine incorporation) at 6 hr, at which time it reaches its maximum (as previously determined). Data are the means ±sd from three replicates. C, control; prot, protein.
(C) Total GSH content in control cells (solid circles), cells treated with 0.1 mM GSH (solid squares), 0.1 mM BSO (open squares), and 1 mM BSO (open circles). Data are the means ±sd from three replicates. gdw, grams dry weight of tissue.
The relatively stable GSH levels during the first 6 hr argues against the hypothesis that de novo synthesis of GSH is required for accomplishment of the G1-to-S transition. Furthermore, the rapid increase in the intracellular GSH concentration triggered by applying GSH (100 μM or 1 mM) to the cells had no effect on the timing or duration of cell cycle phases, as indicated by measurement of DNA synthesis (Figure 5B) and cytometric analysis (Figure 5A). In contrast, application of BSO (100 μM or 1 mM) to the synchronized BY-2 cells resulted in a reduction in the intracellular GSH concentration (Figure 5C), DNA synthesis (Figure 5B), and the proportion of cells in the G2 phase (Figure 5A). It should be noted that some cells appear to be insensitive to BSO treatment and are not blocked in the G1 phase when treated with 100 μM or 1 mM BSO (Figures 5A and 5B). Such cells progress normally through the G2 phase and into mitosis, as measured using the mitotic index (Figure 5 and data not shown), indicating that there is no secondary block during G2 phase. Therefore, we conclude that exit from the G1 phase and entry into S phase require a minimum level of GSH (Figures 5A and 5B).
Downregulation of Cell Cycle Genes at the G1-to-S Phase Transition in Tobacco Cell Suspensions
To study the mechanisms underlying the G1 block induced by depletion of GSH, we investigated the expression of genes encoding two mitotic cyclins, CycA1.1 and CycA3.2, and histone H4. Figure 6 shows that in untreated synchronized cell suspensions, CycA3.2 mRNA started to accumulate at the G1-to-S phase transition at 3 hr, with kinetics similar to H4 mRNA, whereas CycA1.1 mRNA started to accumulate at mid-S phase at 6 to 8 hr (Figure 6). These variations in the transcript amounts for the two cyclins are similar to that previously observed (Reichheld et al., 1996) and suggest a possible role for CycA3.2 in the G1-to-S phase transition and for CycA1.1 during S phase (Figure 6; Reichheld et al., 1996).
Figure 6.
Downregulation of Cell Cycle Genes by Depletion of Intracellular GSH Concentration in Synchronized Tobacco BY-2 Cells.
RNA gel blot hybridization analysis of histone H4 (H4), cyclin A1.1 (CycA1.1), cyclin A3.2 (CycA3.2), and parB (encoding GST) transcripts in synchronized tobacco BY-2 cells. The cells were treated with exogenous GSH (0.1 mM) at the beginning of G1 phase (defined as time 0 of the experiment) or with BSO (0.1 or 1 mM) 2 hr before entry into G1 phase. Control cells were treated with water at time 0. Loading was monitored by ethidium bromide staining of the membrane (rRNA). Similar results were obtained in three different experiments.
To assess directly the impact of changes in the intracellular concentration of GSH on the cell cycle machinery, we investigated whether the temporal pattern of cell cycle gene expression described above was modified by the addition of GSH or BSO. In agreement with the flow cytometric and DNA synthesis analyses (Figures 5A and 5B), exogenous GSH at 100 μM or 1 mM did not modify the expression of the cell cycle genes analyzed (Figure 6 and data not shown). However, depletion of GSH by BSO treatment markedly modified the amplitude but not the timing of cell cycle gene expression (Figure 6). Moreover, the effect of BSO treatment on cell cycle gene expression was dose dependent (Figure 6), suggesting that the intracellular concentration of GSH directly influences cell cycle gene expression, either through direct molecular interaction or by interfering with signal transduction.
We also analyzed the temporal pattern of parB gene expression. parB encodes a tobacco glutathione–S transferase (GST) that has been shown to be induced when tobacco cells enter the cell cycle (Takahashi and Nagata, 1992). Therefore, analysis of parB expression is of interest in this context because the gene encodes a protein implicated in the metabolism of GSH and is also linked to progression through the cell cycle. In control cells, the steady state level of parB mRNA showed marked variations during the cell cycle. parB gene expression is maximal at 6 hr (mid-S phase) (Figure 6), supporting the proposition that the product of this gene may interact functionally with the cell cycle. Exogenous GSH stimulated an increase in the amplitude and the duration of parB transcript accumulation (Figure 6). BSO treatment blocked parB mRNA expression after 3 hr, although treatments of 1 mM BSO led to visibly lower steady state levels of parB mRNA than did treatments of 100 μM (Figure 6). BSO treatment did not modify the expression of other genes whose functions are not related to the cell cycle but related to aspects of cellular redox status (ascorbate peroxidase and Mn superoxide dismutase; data not shown). Depletion of the intracellular concentration of GSH on gene expression thus appears to be specific to cell cycle gene expression rather than to indiscriminate perturbations of transcription.
DISCUSSION
RML1 Encodes the Arabidopsis γ-GCS
We have cloned the Arabidopsis RML1 gene based on its map position. Sequence analysis indicates that it encodes a previously described metabolic enzyme, γ-GCS, which is the first enzyme of GSH biosynthesis (May and Leaver, 1994; Figure 1). In Arabidopsis, γ-GCS is encoded by a single nuclear gene (May and Leaver, 1994). Biosynthesis of GSH in all organisms studied to date occurs in two steps. In the first step, which is catalyzed by γ-GCS, the presumed rate-limiting enzyme in GSH biosynthesis (Cobbett et al., 1998), γ-GC is synthesized from l-glutamate and l-cysteine. The second step, which is catalyzed by GSHS, directly yields GSH and is not affected in the rml1 mutants (Table 1).
The location of the mutation in the rml1 mutants was analyzed in two allelic variants of rml1. These are rml1-1 and rml1-2 (Cheng et al., 1995) that were shown to represent the same substitution at position 258 of the derived amino acid sequence and thus represent the same allele. The mutation is located close to a highly conserved cysteine residue at position 251 in Arabidopsis, which is believed to be part of the active site (Lueder and Phillips, 1996). This region is the only sequence that is closely related in the γ-GCS of Arabidopsis and that of other organisms (May et al., 1998). The absence of detectable γ-GCS activity in rml1 plants strongly supports the proposition that this cysteine residue plays a major role in the enzymatic activity of the Arabidopsis γ-GCS. In agreement with this hypothesis, another mutation in the same gene, cad2-1, is more distant from the essential cysteine residue, and γ-GCS activity is reduced only to 40% in these mutant plants (Cobbett et al., 1998). The rml1 mutation probably modifies the accessibility of the cysteine residue or its reactivity.
Root Phenotype of rml1 Is Specifically Related to GSH
As indicated by the extremely low cellular concentration of GSH and the complete rescue by exogenous GSH (Table 1 and Figure 2), the developmental phenotype observed in rml1 mutants is related to the impairment in GSH biosynthesis. Due to the presence of a strong nucleophilic thiol group on the cysteine residue, GSH is a powerful reductant and is one of the most important low molecular weight antioxidants in plants and other organisms. It is present in high concentrations and has proposed roles in the storage and transport of reduced sulfur, in the synthesis of proteins and nucleic acids, and as a modulator of enzyme activity (May et al., 1998).
Exogenous ascorbate could not rescue the rml1 mutants, despite partially overlapping functions between ascorbate and GSH (May et al., 1998). Similarly, the rml1 mutants could not be rescued by a synthetic thiol, DTT. Thus, the effects on root development observed in rml1 mutants are highly dependent on the intracellular concentration of GSH and not simply on cellular antioxidant capacity. Moreover, shoot growth in rml1 suggests that the developmental effects of the rml1 mutation are not simply due to a metabolic defect, resulting from the impairment of general biochemical processes requiring GSH. Therefore, the rml1 mutation reveals that GSH fulfills more specific developmental functions, which until now have remained obscure despite intense interest in this cellular antioxidant (see below).
Surprisingly, the cad2-1 mutation in γ-GCS does not result in a perturbation of cell division (Howden et al., 1995). However, in this mutant, the intracellular concentration of GSH is 15 to 30% of that in the wild type (Cobbett et al., 1998), whereas it is only 2.7% in rml1 (Table 1). In the absence of further mutations, we can thus assume that the physiological differences between these two mutants are directly linked to differences in the cellular concentration of GSH. A threshold concentration must therefore exist, below which developmental effects are observed in the root. The existence of this threshold suggests that in wild-type Arabidopsis, GSH is in large excess or that some compensatory mechanisms exist that become insufficient when the intracellular GSH concentration is too low, reinforcing the idea that rml1 mutants are affected in a developmental process specifically requiring GSH.
A GSH-Dependent Developmental Pathway Controls Cell Division in the Postembryonic Root
A few genes that affect cell division in shoot meristems have been cloned, such as SHOOT MERISTEMLESS (STM) and CLAVATA1 and 3 (CLV1 and CLV3), that could be involved in signal transduction pathways (Meyerowitz, 1997). The specific effect of the rml1 mutation on cell division in the postembryonic root has suggested that RML1 could be such a protein (Cheng et al., 1995). The identity of RML1 as γ-GCS, which is a metabolic enzyme, is thus rather surprising. However, we have shown that in two different plant systems, Arabidopsis and tobacco, depletion of intracellular GSH, which is the direct effect of the rml1 mutation, completely inhibits cell division in the root. From the phenotype of rml1 Arabidopsis plants and from the specific cell cycle block induced in tobacco cell suspension by depletion of intracellular GSH (Figures 4C and 5), the rml1 mutation has a clear effect on cell cycle progression; thus, an adequate intracellular concentration of GSH is required for normal progression through the cell cycle.
rml1 seedlings can produce leaves and flowers (Cheng et al., 1995), suggesting that progression through the cell cycle is not seriously affected in the shoot apical meristem. This indicates that the rml1 mutation (or depletion of intracellular GSH) does not affect division of all plant cells. A possible indirect effect of GSH on root cell cycle may be through modulation of upstream signal transduction processes. Because the oxidized form of GSH, GSSG, could not rescue the rml1 mutants, the effects of GSH on root development are most probably derived from the reducing capacity of the thiol. Indeed, redox mechanisms function in the regulation of transduction pathways in animals. A number of transcription factors have been reported that show redox-dependent changes in their ability to bind DNA (Abate et al., 1990; Toledano and Leonard, 1991; Mihm et al., 1995). Moreover, activation of the animal cell cycle in response to growth factors appears to be modulated by the intracellular GSH concentration (Liang et al., 1989; Suthantiran et al., 1990), suggesting the existence of GSH-dependent transduction pathways in mammalian cells.
The absence of cell cycle activity in the postembryonic roots of rml1 mutants suggests the existence of a similar GSH-dependent transduction pathway that regulates the cell cycle in the postembryonic root and may indicate that this is a general mechanism in all organisms. GSH biosynthesis appears necessary to activate and maintain cell division in the postembryonic root. Such a GSH-dependent pathway would then play a role not only in activation but also in maintenance of cell cycle activity in the root after germination. Alternatively, GSH may not directly affect the redox state of target developmental regulators. Some GSTs have been shown to be regulated during the cell cycle (e.g., parB; Nagata et al., 1992; this study). GSH-dependent modulation of the cell cycle activity during postembryonic root development could involve specific GSH conjugates through a non-redox mechanism, although implication of GSTs in developmental processes clearly awaits experimental demonstration.
The fact that the rml1 mutation affects primarily postembryonic cell division in the root suggests that the embryo receives GSH from the maternal tissue; in the postembryonic shoot, putative GSH-dependent factors, if present, are regulated by other redox-active molecules. However, we cannot exclude that differences in sensitivity to GSH between the root and the shoot may account for the root specificity of the rml1 mutation. Clearly, at this stage, the isolation of other mutants defective in the RML1 gene, and notably of a mutant synthesizing no GSH (if not lethal), is an indispensable step to define precisely the function of GSH in root development.
GSH-Dependent Control of the G1-to-S Phase Transition
Quantification of the nuclear DNA content of cells in the rml1 root tip did not allow us to identify a block in cell cycle–specific gene expression due to the presence of endoreduplicated cells (Figure 3). Given the absence of DNA synthesis after germination (Cheng et al., 1995), this situation must reflect embryonic events that are influenced by maternally supplied GSH. However, because drug-mediated depletion of GSH in tobacco cell suspensions blocked entry into S phase, the physiological impact of the rml1 lesion is likely also to be manifested as a G1 block in postembryonic roots. The depletion of intracellular GSH in tobacco cell suspension downregulated two different A-type cyclins that are putatively involved in the G1-to-S phase transition and in progression into S phase (Reichheld et al., 1996; Figure 6). Cyclins have been shown to be potential targets for growth control in plants (Doerner et al., 1996), and the downregulation of these two cyclins therefore could be partially responsible for the specific G1 block induced by depletion of intracellular GSH rather than being simply the result of the cell cycle arrest or of a diminution of the population of cycling cells.
Cyclin-dependent kinase inhibitor (CKI) activation also could account for the G1 block induced by depletion of intracellular GSH. p21 is a CKI that was shown to be involved in mammalian cells in the negative regulation of the G1-to-S transition (Elledge et al., 1996). Using the GSH-depleting drug diethyl maleate, Russo et al. (1995) observed a dose-dependent induction of the p21 protein in mammalian cells. This induction was concomitant with a G1 block. Therefore, depletion of intracellular GSH could block root cells in the G1 phase in a similar fashion. The description of the first CKI in plants (Wang et al., 1997) and the availability of mutants, such as rml1, coupled with the amenability of the BY-2 cell suspension, clearly offer tools to dissect the molecular mechanisms underlying the G1 block induced by depletion of intracellular GSH.
METHODS
Plant Growth Conditions, Strains, and Treatments
Unless stated otherwise, Arabidopsis thaliana ecotype Columbia plants (root meristemless1 [rml1] and the wild type) were grown as described in Cheng et al. (1995). Supplementation of the growth medium with γ-glutamylcysteine (γ-GC), glutathione (GSH), the GSH oxidized form (GSSG), l-buthionine-(S,R)-sulfoximine (BSO), DTT, ascorbate, and glutamate and cysteine (Sigma) at various concentrations was performed as follows. Appropriate amounts of filter-sterilized aqueous stock solutions containing these substances were added to sterile Petri dishes, and molten two-fifths Murashige Skoog (MS) medium (Murashige and Skoog, 1962) that had been cooled to 48°C was added while swirling the plates. After plating surface-sterilized seeds, the plates were stored in the dark at 4°C for 4 days before placing them in nearly vertical racks (∼15° angle) at 21°C under short-day (8 hr of light/16 hr of darkness) conditions. Seedlings were photographed with a single-lens reflex camera (model 8000S; Nikon Corporation, Tokyo, Japan).
Nicotiana tabacum cv Petit Havana (SR1) seeds were grown in the presence and absence of BSO as described above. After plating surface-sterilized seeds, the plates were placed at 24°C under long-day (16 hr of light/8 hr of darkness) conditions and photographed with a 35-mm camera (Olympus Optical Company, Tokyo, Japan).
Tobacco BY-2 Cell Suspension and Synchronization
A tobacco Bright Yellow 2 (BY-2) cell culture was maintained as described by Nagata et al. (1992). For experiments with exponentially growing BY-2 cells, 3-day-old suspensions were used. For experiments with synchronized cells, synchronization was performed as described by Reichheld et al. (1996). Briefly, sequential treatment with 3 μg mL−1 aphidicolin (an inhibitor of DNA synthesis; Sigma) and then with 1.54 μg mL−1 propyzamide (an antitubulin drug; Sumitomo Chemical Company, Tokyo, Japan) was used to study the G1-to-S phase transition specifically. For treatment with exogenous GSH or BSO at various concentrations at the G1-to-S phase transition, GSH was added at the indicated concentration 2 hr after the removal of propyzamide (this later point being time 0 of the experiment); BSO was added directly after the removal of the propyzamide. DNA synthesis was measured as described by Reichheld et al. (1996).
Positional Cloning and Sequencing of RML1
Generation of the segregating F2 mapping population and restriction fragment length polymorphism (RFLP) mapping were performed as previously described (Cheng et al., 1995). Isolation of yeast artificial chromosome (YAC) ends and subsequent detection of ecotype-specific RFLPs were as described in Schmidt et al. (1995). To construct the T-DNA transformation-ready cosmid library, total DNA isolated from the yeast strain harboring the RML1-spanning YAC clone CIC5A4 was partially digested with MboI and size-fractionated over a sucrose gradient to obtain 20- to 25-kb fragments. These fragments were then ligated to BamHI-digested pCLD04541 vector DNA and transfected into Escherichia coli.
Approximately 3000 recombinant clones were obtained, and these were cultured in 96-well microtiter plates, lifted onto nylon membranes (Hybond; Amersham) for screening, and then stored as glycerol stocks at −80°C. End fragments from the cosmids of interest were identified by electrophoresing DNA restricted with SacI, ClaI, and both enzymes. Each left a 30- to 40-bp polylinker tag on the insert end fragments; the resulting gels were transferred to nylon membranes and hybridized with each of two radiolabeled PvuII-BamHI fragments derived from the polylinker of pCLD04541.
We defined cosmid end fragments flanking the ClaI site as left-end fragments and those flanking the SacI site as right-end fragments. Cosmids were mobilized into Agrobacterium tumefaciens by electroporation according to the manufacturer's instructions (Bio-Rad). Heterozygous rml1 plants were transformed with Agrobacterium by the vacuum infiltration procedure (Bechtold et al., 1993); the resulting T1 seed was harvested from individual infiltrated plants and selected on two-fifths MS medium containing kanamycin at 50 μg mL−1. As a control, transformation was also performed with Agrobacterium harboring an empty pCLD04541 plasmid. The kanamycin-resistant T1 seedlings from plants also segregating rml1 were selfed, and the resulting T2 seedlings were scored for both kanamycin resistance and the Rml1 phenotype. Insert DNA from the two complementing cosmids, 20A7 and 26A8, were radiolabeled and used to screen ∼300,000 λ-Prl2 cDNA clones (stock No. CD4-7; Arabidopsis Biological Resource Center, Columbus, OH) according to established protocols (Sambrook et al., 1989). Two cDNA clones hybridizing to both cosmids were analyzed by restriction digestion and partial sequencing.
Genomic DNA was sequenced as described previously (Cobbett et al., 1998). The GenBank accession number for RML1/CAD2 is AF068299.
Thiol Determination and Enzyme Activities
Total GSH content of BY-2 cells was determined using the recycling enzymatic assay, as described by May and Leaver (1993). γ-GC, cysteine, and total GSH, γ-GCS, and GSH synthetase (GSHS) activities in rml1 plants were measured, as described by Cobbett et al. (1998), from liquid-grown Arabidopsis tissues (May and Leaver, 1993). Protein was determined according to the method of Lowry, with modifications by Peterson (1977).
Flow Cytometry
For flow cytometric analysis, protoplasts of tobacco BY-2 cells were prepared for 1 hr with 2% cellulase Onozuka R10 and 0.1% pectolyase (Kikkoman Company, Tokyo, Japan). The cells were incubated at 37°C, washed, and lysed in Galbraith's buffer (Galbraith et al., 1983), filtered in 1% formaldehyde through 10-μm nylon mesh, treated with RNase A, and stained with propidium iodide (50 μg mL−1). Cytometric analysis was performed using 104 nuclei on an EPICS flow cytometer (Beckman Coulter, Roissy, France). For flow cytometric analysis of nuclear DNA content in roots, 10 to 30 root tips were chopped with a razor blade in Galbraith's extraction buffer (Galbraith et al., 1983) and analyzed as described by Gendreau et al. (1998). For all results discussed in the text, two populations were estimated as significantly different if their deviation exceeded 5%.
RNA Analysis
Total RNA was extracted using Trizol according to the manufacturer's instructions (Boehringer Mannheim). RNA gel blot analysis was performed essentially as described by Reichheld et al. (1996), and radioactively labeled fragments corresponding to the coding region of H4A748 (histone 4 cDNA clone A748) from Arabidopsis (Chabouté et al., 1987), parB (Takahashi and Nagata, 1992), and CycA1.1 and CycA3.2 (Reichheld et al., 1996) from tobacco were used as probes.
Acknowledgments
We thank Beatrice Satiat-Jeunemaitre, Jan Traas, and Mary Alice Yund for comments on the work and critical reading of the manuscript. The cosmid vector pCLD04541 was kindly provided by Brian Staskawicz, YAC clones by Caroline Dean, and parB cDNA by Toshiyuki Nagata. This work was supported by a National Science Foundation grant (No. IBN-9513522) to Z.R.S. and by grants from the Belgian Program on Interuniversity Poles of Attraction (Prime Minister's Office, Science Policy Programming and Grant No. 38) and the Vlaams Actieprogramma Biotechnologie (No. ETC 002) to M.V.M. T.V. is recipient of Rhône-Poulenc Agrochimie and the Institut de la Recherche Agronomique for predoctoral training grants; R.C.W. received a U.S. Department of Agriculture postdoctoral grant (No. 95-37305-2299); J.-P.R. received a grant from the European Commission, Research Training Project (No. ERBFMBI-CT96-1274); M.J.M. is a recipient of the European Molecular Biology Organization for a postdoctoral fellowship; and C.S.C. was supported by a grant from the Australian Research Council.
References
- Abate, C., Patel, L., Rauscher, F.J., and Curran, T. (1990). Redox regulation of Fos and Jun DNA-binding activity in vitro. Science 249 1157–1161. [DOI] [PubMed] [Google Scholar]
- Bechtold, N., Ellis, J., and Pelletier, G. (1993). In planta Agrobacterium-mediated gene transfer by infiltration of adult Arabidopsis thaliana plants. C. R. Acad. Sci. Ser. III Sci. Vie 316 1194–1199. [Google Scholar]
- Bent, A.F., Kunkel, B.N., Dahlbeck, D., Brown, K.L., Schmidt, R., Giraudat, J., Leung, J., and Staskawicz, B.J. (1994). RPS2 of Arabidopsis thaliana: A leucine-rich repeat class of plant disease resistance genes. Science 265 1856–1860. [DOI] [PubMed] [Google Scholar]
- Chabouté, M.E., Chaubet, N., Philipps, G., Ehling, M., and Gigot, C. (1987). Genomic organisation and nucleotide sequences of two histone H3 and two histone H4 genes of Arabidopsis thaliana. Plant Mol. Biol. 17 179–191. [DOI] [PubMed] [Google Scholar]
- Cheng, J.C., Seeley, K., and Sung, Z.R. (1995). RML1 and RML2, Arabidopsis genes required for cell proliferation at the root tip. Plant Physiol. 107 365–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobbett, C.S., May, M.J., Howden, R., and Rolls, B. (1998). The glutathione-deficient, cadmium-sensitive mutant, cad2-1, of Arabidopsis thaliana is deficient in γ-glutamylcysteine synthetase. Plant J. 16 73–78. [DOI] [PubMed] [Google Scholar]
- Doerner, P., Jørgensen, J.-E., You, R., Steppuhn, J., and Lamb, C.J. (1996). Control of root growth and development by cyclin expression. Nature 380 520–523. [DOI] [PubMed] [Google Scholar]
- Dolan, L., Janmaat, K., Willemsen, V., Linstead, P., Poethig, S., Roberts, K., and Scheres, B. (1993). Cellular organization of the Arabidopsis thaliana root. Development 119 71–84. [DOI] [PubMed] [Google Scholar]
- Elledge, S.J., Winston, J., and Harper, J.W. (1996). A question of balance: The role of cyclin-kinase inhibitors in development and tumorigenesis. Trends Cell Biol. 6 388–392. [DOI] [PubMed] [Google Scholar]
- Galbraith, D.W., Harkins, K.R., Maddox, J.R., Ayres, N.M., Sharma, D.P., and Firoozabady, E. (1983). Rapid flow cytometry analysis of the cell cycle in intact plant tissues. Science 250 99–101. [DOI] [PubMed] [Google Scholar]
- Galbraith, D.W., Harkins, K.R., and Knapp, S. (1991). Systemic endopolyploidy in Arabidopsis thaliana. Plant Physiol. 96 985–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gendreau, E., Höfte, H., Grandjean, O., Brown, S., and Traas, J. (1998). Phytochrome controls the number of endoreduplication cycles in the Arabidopsis thaliana hypocotyl. Plant J. 13 221–230. [DOI] [PubMed] [Google Scholar]
- Griffith, O.W., and Meister, A. (1979). Potent and specific inhibition of glutathione synthesis by buthionine sulfoximine (S-n butyl homocysteine sulfoximine). J. Biol. Chem. 254 7558–7560. [PubMed] [Google Scholar]
- Hemerly, A., de Almeida Engler, J., Bergounioux, C., Van Montagu, M., Engler, G., Inzé, D., and Ferreira, P. (1995). Dominant negative mutants of Cdc2 kinase uncouple cell division from iterative plant development. EMBO J. 14 3925–3936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howden, R., Andersen, C.R., Goldsbrough, P.B., and Cobbett, C.S. (1995). Cadmium-sensitive, glutathione-deficient mutant of Arabidopsis thaliana. Plant Physiol. 107 1067–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laux, T., and Jürgens, G. (1997). Embryogenesis: A new start in life. Plant Cell 9 989–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang, C.M., Lee, N., Cattell, D., and Liang, S.M. (1989). Glutathione regulates interleukin-2 activity on cytotoxic T-cells. J. Biol. Chem. 264 13519–13523. [PubMed] [Google Scholar]
- Lueder, D.V., and Phillips, M.A. (1996). Characterization of Tryponosoma brucei γ-glutamylcysteine synthetase, an essential enzyme in the biosynthesis of trypanothione (diglutathionylspermidine). J. Biol. Chem. 271 17485–17490. [DOI] [PubMed] [Google Scholar]
- May, M.J., and Leaver, C.J. (1993). Oxidative stimulation of glutathione synthesis in Arabidopsis thaliana suspension cultures. Plant Physiol. 103 621–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May, M.J., and Leaver, C.J. (1994). Arabidopsis thaliana γ-glutamylcysteine synthetase is structurally unrelated to mammalian, yeast and E. coli homologs. Proc. Natl. Acad. Sci. USA 91 10059–10063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May, M.J., Vernoux, T., Leaver, C.J., Van Montagu, M., and Inzé, D. (1998). Glutathione homeostasis in plants: Implications for environmental sensing and plant development. J. Exp. Bot. 49 649–667. [Google Scholar]
- Meyerowitz, E.M. (1997). Genetic control of cell division pattern in developing plants. Cell 88 299–308. [DOI] [PubMed] [Google Scholar]
- Mihm, S., Galter, D., and Dröge, W. (1995). Modulation of transcription factor NFκ-B activity by intracellular glutathione levels and by variations of the extracellular cysteine supply. FASEB J. 9 246–252. [DOI] [PubMed] [Google Scholar]
- Murashige, T., and Skoog, F. (1962). A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 15 473–497. [Google Scholar]
- Nagata, T., Nemoto, Y., and Hasezawa, S. (1992). Tobacco BY-2 cell line as the “HeLa” cell in the cell biology of higher plants. Int. Rev. Cytol. 132 1–30. [Google Scholar]
- Peterson, G.L. (1977). A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal. Biochem. 83 346–356. [DOI] [PubMed] [Google Scholar]
- Reichheld, J.-P., Chaubet, N., Shen, W.H., Renaudin, J.-P., and Gigot, C. (1996). Multiple A-type cyclins express sequentially during the cell cycle in Nicotiana tabacum BY-2 cells. Proc. Natl. Acad. Sci. USA 93 13819–13824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo, T., Zambrano, N., Esposito, F., Ammendola, R., Cimino, F., Fiscella, M., Jackman, J., O'Connor, M., Anderson, C.W., and Apella, E. (1995). A p53-independent pathway for activation of WAF1/CIP1 expression following oxidative stress. J. Biol. Chem. 270 29386–29391. [DOI] [PubMed] [Google Scholar]
- Sambrook, J., Fritsch, E.F., and Maniatis, T. (1989). Molecular Cloning: A Laboratory Manual, 2nd ed. (Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press).
- Sánchez-Fernández, R., Fricker, M., Corben, L.B., White, N.S., Sheard, N., Leaver, C.J., Van Montagu, M., Inzé, D., and May, M.J. (1997). Cell proliferation and hair tip growth in the Arabidopsis root are under mechanistically different forms of redox control. Proc. Natl. Acad. Sci. USA 94 2745–2750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheres, B., Wolkenfelt, H., Willemsen, V., Terlouw, M., Lawson, E., Dean, C., and Weisbeek, P. (1994). Embryonic origin of the Arabidopsis primary root and root meristem initials. Development 120 2475–2487. [Google Scholar]
- Scheres, B., McKahnn, H.I., and Van den Berg, C. (1996). Roots redefined: Anatomical and genetic analysis of root development. Plant Physiol. 111 959–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiefelbein, J.W., Masucci, J.D., and Wang, H. (1997). Building a root: The control of patterning and morphogenesis during root development. Plant Cell 9 1089–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt, R., West, J., Lenehan, Z., Lister, C., Thompson, H., Bouchez, D., and Dean, C. (1995). Physical map and organization of Arabidopsis thaliana chromosome 4. Science 270 480–483. [DOI] [PubMed] [Google Scholar]
- Suthantiran, M., Anderson, M.E., Sharma, V.K., and Meister, A. (1990). Glutathione regulates activation-dependent DNA synthesis in highly purified normal human T lymphocytes via the CD2 and CD3 antigens. Proc. Natl. Acad. Sci. USA 87 3343–3347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi, Y., and Nagata, T. (1992). An auxin-regulated gene encoding glutathione S-transferase. Proc. Natl. Acad. Sci. USA 89 56–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toledano, M.B., and Leonard, W.J. (1991). Modulation of transcription factor NFκ-B binding activity by oxidation reduction in vitro. Proc. Natl. Acad. Sci. USA 88 4328–4332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Berg, C., Willemsen, V., Hage, W., Weisbeek, P., and Scheres, B. (1995). Cell fate in the Arabidopsis root meristem by directional signaling. Nature 378 62–65. [DOI] [PubMed] [Google Scholar]
- Van den Berg, C., Willemsen, V., Hendriks, G., Weisbeek, P., and Scheres, B. (1997). Short-range control of cell differentiation in the Arabidopsis root meristem. Nature 390 287–289. [DOI] [PubMed] [Google Scholar]
- Wang, H., Fowke, L.C., and Crosby, W.L. (1997). A plant cyclin–dependent kinase inhibitor gene. Nature 386 451–452. [DOI] [PubMed] [Google Scholar]